Abstract
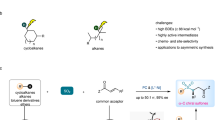
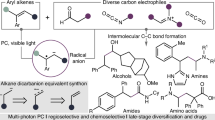
The selective functionalization of inert C(sp3)–H bonds is extremely attractive in organic synthesis and catalysis science, but the conversion of hydrocarbons lacking directing groups into chiral molecules through catalytic C(sp3)–H functionalization is formidably challenging. Here, to address this problem, we have developed a photochemical system consisting of a hydrogen atom transfer organophotocatalyst and a chiral catalyst containing an earth-abundant metal. With the cooperative catalysts and imine partners, a wide range of benzylic, allylic hydrocarbons and unactivated alkanes can be converted to functionalized chiral products. The readily tunable bisoxazoline catalysts of copper or other metals exhibit precise regional recognition and asymmetric induction towards these inert C–H bonds. The reactions are applicable to many compounds including small hydrocarbons, branched alkanes, cycloalkanes and more complex medicinal agents. This method provides an economic and rapid construction of optically active compounds, starting from the most basic chemical feedstocks.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings of this study are available from the corresponding author upon request.
References
Labinger, J. A. & Bercaw, J. E. Understanding and exploiting C–H bond activation. Nature 417, 507–514 (2002).
Caballero, A. et al. Silver-catalyzed C–C bond formation between methane and ethyl diazoacetate in supercritical CO2. Science 332, 835–838 (2011).
Cook, A. K., Schimler, S. D., Matzger, A. J. & Sanford, M. S. Catalyst-controlled selectivity in the C–H borylation of methane and ethane. Science 351, 1421–1424 (2016).
Zhang, Rj. K. et al. Enzymatic assembly of carbon–carbon bonds via iron-catalysed sp 3 C–H functionalization. Nature 565, 67–72 (2018).
Prier, C. K., Zhang, R. K., Buller, A. R., Brinkmann-Chen, S. & Arnold, F. H. Enantioselective, intermolecular benzylic C–H amination catalysed by an engineered iron-haem enzyme. Nat. Chem. 9, 629–634 (2017).
Cuthbertson, J. D. & MacMillan, D. W. C. The direct arylation of allylic sp 3 C–H bonds via organic and photoredox catalysis. Nature 519, 74–77 (2015).
Tran, B. L., Li, B., Driess, M. & Hartwig, J. F. Copper-catalyzed intermolecular amidation and imidation of unactivated alkanes. J. Am. Chem. Soc. 136, 2555–2563 (2014).
Mukherjee, S., Maji, B., Tlahuext-Aca, A. & Glorius, F. Visible-light-promoted activation of unactivated C(sp 3)–H bonds and their selective trifluoromethylthiolation. J. Am. Chem. Soc. 138, 16200–16203 (2016).
Sharma, A. & Hartwig, J. F. Metal-catalysed azidation of tertiary C–H bonds suitable for late-stage functionalization. Nature 517, 600–604 (2015).
Tang, S., Wang, P., Li, H. & Lei, A. Multimetallic catalysed radical oxidative C(sp 3)–H/C(sp)–H cross-coupling between unactivated alkanes and terminal alkynes. Nat. Commun. 7, 11676 (2016).
Clark, J. R., Feng, K., Sookezian, A. & White, M. C. Manganese-catalysed benzylic C(sp3)–H amination for late-stage functionalization. Nat. Chem. 10, 583–591 (2018).
Hu, A., Guo, J. J., Pan, H. & Zuo, Z. Selective functionalization of methane, ethane, and higher alkanes by cerium photocatalysis. Science 361, 668–672 (2018).
Margrey, K. A., Czaplyski, W. L., Nicewicz, D. A. & Alexanian, E. J. A general strategy for aliphatic C–H functionalization enabled by organic photoredox catalysis. J. Am. Chem. Soc. 140, 4213–4217 (2018).
Yi, H. et al. Recent advances in radical C–H activation/radical cross-coupling. Chem. Rev. 117, 9016–9085 (2017).
Saint–Denis, T. G., Zhu, R. –Y., Chen, G., Wu, Q. –F. & Yu, J. –Q. Enantioselective C(sp 3)–H bond activation by chiral transition metal catalysts. Science 359, eaao4798 (2018).
Rouquet, G. & Chatani, N. Catalytic functionalization of C(sp2)–H and C(sp3)–H bonds using bidentate directing groups. Angew. Chem. Int. Ed. 52, 11726–11743 (2013).
He, J. et al. Ligand-controlled C(sp3)–H arylation and olefination in synthesis of unnatural chiral α-amino acids. Science 343, 1216–1220 (2014).
Zhang, F. –L., Hong, K., Li, T. –J., Park, H. & Yu, J. –Q. Functionalization of C(sp 3)–H bonds using a transient directing group. Science 351, 252–256 (2016).
Choi, G. J., Zhu, Q., Miller, D. C., Gu, C. J. & Knowles, R. R. Catalytic alkylation of remote C–H bonds enabled by proton-coupled electron transfer. Nature 539, 268–271 (2016).
Chu, J. C. K. & Rovis, T. Amide-directed photoredox-catalysed C–C bond formation at unactivated sp 3 C–H bonds. Nature 539, 272–275 (2016).
Liao, K. et al. Site-selective and stereoselective functionalization of nonactivated tertiary C–H bonds. Nature 551, 609–613 (2017).
Liao, K., Negretti, S., Musaev, D. G., Bacsa, J. & Davies, H. M. L. Site-selective and stereoselective functionalization of unactivated C–H bonds. Nature 533, 230–234 (2016).
Liao, K. et al. Design of catalysts for site-selective and enantioselective functionalization of non-activated primary C–H bonds. Nat. Chem. 10, 1048–1055 (2018).
Fu, J. T., Ren, Z., Bacsa, J., Musaev, D. G. & Davies, H. M. L. Desymmetrization of cyclohexanes by site- and stereoselective C–H functionalization. Nature 564, 395–399 (2018).
Mazzarella, D., Crisenza, G. E. M. & Melchiorre, P. Asymmetric photocatalytic C−H functionalization of toluene and derivatives. J. Am. Chem. Soc. 140, 8439–8443 (2018).
Zhang, W. et al. Enantioselective cyanation of benzylic C–H bonds via copper catalyzed radical relay. Science 353, 1014–1018 (2016).
Li, F. et al. Chiral acid-catalysed enantioselective C–H functionalization of toluene and its derivatives driven by visible light. Nat. Commun. 10, 1774 (2019).
Narayanam, J. M. R. & Stephenson, C. R. J. Visible light photoredox catalysis: applications in organic synthesis. Chem. Soc. Rev. 40, 102–113 (2011).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013).
Chen, J. –R., Hu, X. –Q., Lu, L. –Q. & Xiao, W. –J. Visible light photoredoxcontrolled reactions of N-radicals and radical ions. Chem. Soc. Rev. 45, 2044–2056 (2016).
Perry, I. B. et al. Direct arylation of strong aliphatic C–H bonds. Nature 560, 70–75 (2018).
Heitz, D. R., Tellis, J. C. & Molander, G. A. Photochemical nickel-catalyzed C–H arylation: synthetic scope and mechanistic investigations. J. Am. Chem. Soc. 138, 12715–12718 (2016).
Ackerman, L. K. G., Alvarado, J. I. M. & Doyle, A. G. Direct C−C bond formation from alkanes using Ni-photoredox catalysis. J. Am. Chem. Soc. 140, 14059–14063 (2018).
Shen, Y., Gu, Y. & Martin, R. sp3 C–H arylation and alkylation enabled by the synergy of triplet excited ketones and nickel catalysts. J. Am. Chem. Soc. 140, 12200–12209 (2018).
Fan, X. –Z. et al. Eosin Y as a direct hydrogen-atom transfer photocatalyst for the functionalization of C–H bonds. Angew. Chem. Int. Ed. 57, 8514–8518 (2018).
Dewanji, A., Krach, P. E. & Rueping, M. The dual role of benzophenone in visible-light/nickel photoredox-catalyzed C−H arylations: hydrogen-atom transfer and energy transfer. Angew. Chem. Int. Ed. 58, 3566–3570 (2019).
Xia, J.-B., Zhu, C. & Chen, C. Visible light-promoted metal-free C–H activation: diarylketone-catalyzed selective benzylic mono- and difuorination. J. Am. Chem. Soc. 135, 17494–17500 (2013).
Kamijo, S., Kamijo, K., Maruoka, K. & Murafuji, T. Aryl ketone catalyzed radical allylation of C(sp3)−H bonds under photoirradiation. Org. Lett. 18, 6516–6519 (2016).
Li, Y. et al. Copper(II)-catalyzed asymmetric photoredox reactions: enantioselective alkylation of imines driven by visible light. J. Am. Chem. Soc. 140, 15850–15858 (2018).
Shen, X. et al. A chiral nickel DBFOX complex as a bifunctional catalyst for visible-light-promoted asymmetric photoredox reactions. Chem. Sci. 9, 4562–4568 (2018).
Ma, J. –A. Recent developments in the catalytic asymmetric synthesis of α- and β-amino acids. Angew. Chem. Int. Ed. 42, 4290–4299 (2003).
Ravelli, D., Fagnoni, M., Fukuyama, T., Nishikawa, T. & Ryu, I. Site-selective C−H functionalization by decatungstate anion photocatalysis: synergistic control by polar and steric effects expands the reaction scope. ACS Catal. 8, 701–713 (2018).
Wang, F. –L. et al. Catalytic asymmetric radical diamination of alkenes. Chem 3, 979–990 (2017).
Blanksby, S. J. & Ellison, G. B. Bond dissociation energies of organic molecules. Acc. Chem. Res. 36, 255–263 (2003).
Liu, W. et al. Catalyst-controlled selective functionalization of unactivated C−H bonds in the presence of electronically activated C−H bonds. J. Am. Chem. Soc. 140, 12247–12255 (2018).
Qin, C. & Davies, H. M. L. Role of sterically demanding chiral dirhodium catalysts in site-selective C−H functionalization of activated primary C−H bonds. J. Am. Chem. Soc. 136, 9792–9796 (2014).
Davies, H. M. L. & Hansen, T. Asymmetric intermolecular carbenoid C–H insertions catalyzed by rhodium(II) (S)-N-(p-dodecylphenyl)sulfonylprolinate. J. Am. Chem. Soc. 119, 9075–9076 (1997).
Cundari, T. R. et al. Copper-catalyzed C(sp3)–H amidation: sterically driven primary and secondary C–H site-selectivity. Angew. Chem. Int. Ed. 58, 3421–3425 (2019).
Yoon, T. P. Photochemical stereocontrol using tandem photoredox–chiral Lewis acid catalysis. Acc. Chem. Res. 49, 2307–2315 (2016).
Bartok, M. Unexpected inversions in asymmetric reactions: reactions with chiral metal complexes, chiral organocatalysts, and heterogeneous chiral catalysts. Chem. Rev. 110, 1663–1705 (2010).
Burk, M. J., Allen, J. G. & Kiesman, W. F. Highly regio- and enantioselective catalytic hydrogenation of enamides in conjugated diene systems: synthesis and application of γ,δ-unsaturated amino acids. J. Am. Chem. Soc. 120, 657–663 (1998).
Acknowledgements
We gratefully acknowledge funding from the National Natural Science Foundation of China (grant no. 21572184), the Natural Science Foundation of Fujian Province of China (grant no. 2017J06006) and the Fundamental Research Funds for the Central Universities (grant no. 20720190048).
Author information
Authors and Affiliations
Contributions
L.G. and Y.L. conceived and designed the project. Y.L. and M.L. conducted the experiments. Y.L., M.L. and L.G. analysed and interpreted the experimental data. L.G. prepared the manuscript. Y.L. prepared the Supplementary Information.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, Supplementary Figs. 1–77, Supplementary Tables 1–3 and Supplementary References.
Rights and permissions
About this article
Cite this article
Li, Y., Lei, M. & Gong, L. Photocatalytic regio- and stereoselective C(sp3)–H functionalization of benzylic and allylic hydrocarbons as well as unactivated alkanes. Nat Catal 2, 1016–1026 (2019). https://doi.org/10.1038/s41929-019-0357-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-019-0357-9
This article is cited by
-
Deoxygenative photochemical alkylation of secondary amides enables a streamlined synthesis of substituted amines
Nature Communications (2025)
-
A modular approach to catalytic stereoselective synthesis of chiral 1,2-diols and 1,3-diols
Nature Communications (2025)
-
Nickel-catalysed enantioselective alkene dicarbofunctionalization enabled by photochemical aliphatic C–H bond activation
Nature Catalysis (2024)
-
Modular α-tertiary amino ester synthesis through cobalt-catalysed asymmetric aza-Barbier reaction
Nature Chemistry (2024)
-
Challenges and recent advancements in the synthesis of α,α-disubstituted α-amino acids
Nature Communications (2024)