Abstract
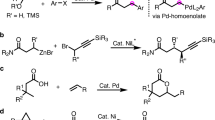
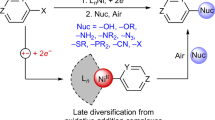
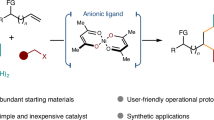
The selective cross-coupling of two alkyl electrophiles to construct complex molecules remains a challenge in organic synthesis1,2. Known reactions are optimized for specific electrophiles and are not amenable to interchangeably varying electrophilic substrates that are sourced from common alkyl building blocks, such as amines, carboxylic acids and halides3,4,5. These limitations restrict the types of alkyl substrate that can be modified and, ultimately, the chemical space that can be explored6. Here we report a general solution to these limitations that enables a combinatorial approach to alkyl–alkyl cross-coupling reactions. This methodology relies on the discovery of unusually persistent Ni(alkyl) complexes that can be formed directly by oxidative addition of alkyl halides, redox-active esters or pyridinium salts. The resulting alkyl complexes can be isolated or directly telescoped to couple with a second alkyl electrophile, which represent cross-selective reactions that were previously unknown. The utility of this synthetic capability is showcased in the rapid diversification of amino acids, natural products, pharmaceuticals and drug-like building blocks by various combinations of dehalogenative, decarboxylative or deaminative coupling. In addition to a robust scope, this work provides insights into the organometallic chemistry of synthetically relevant Ni(alkyl) complexes through crystallographic analysis, stereochemical probes and spectroscopic studies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All experimental data, analytical procedures, cell designs, copies of spectra and CIF data are available in Supplementary Information.
References
Tasker, S. Z., Standley, E. A. & Jamison, T. F. Recent advances in homogeneous nickel catalysis. Nature 509, 299–309 (2014).
Wang, X., Dai, Y. & Gong, H. Nickel-catalyzed reductive couplings. Top. Curr. Chem. 374, 43 (2016).
Yue, H. et al. Nickel-catalyzed C–N bond activation: activated primary amines as alkylating reagents in reductive cross-coupling. Chem. Sci. 10, 4430–4435 (2019).
Huihui, K. M. M. et al. Decarboxylative cross-electrophile coupling of N-hydroxyphthalimide esters with aryl iodides. J. Am. Chem. Soc. 138, 5016–5019 (2016).
Li, P. et al. Nickel-electrocatalysed C(sp3)–C(sp3) cross-coupling of unactivated alkyl halides. Nat. Catal. https://doi.org/10.1038/s41929-024-01118-3 (2024).
Lyon, W. L. & MacMillan, D. W. C. Expedient access to underexplored chemical space: deoxygenative C(sp3)–C(sp3) cross-coupling. J. Am. Chem. Soc. 145, 7736–7742 (2023).
Weix, D. J. Methods and mechanisms for cross-electrophile coupling of Csp2 halides with alkyl electrophiles. Acc. Chem. Res. 48, 1767–1775 (2015).
Truesdell, B. L., Hamby, T. B. & Sevov, C. S. General C(sp2)–C(sp3) cross-electrophile coupling reactions enabled by overcharge protection of homogeneous electrocatalysts. J. Am. Chem. Soc. 142, 5884–5893 (2020).
Hamby, T. B., LaLama, M. J. & Sevov, C. S. Controlling Ni redox states by dynamic ligand exchange for electroreductive Csp3–Csp2 coupling. Science 376, 410–416 (2022).
Zhang, P., Le, C. C. & MacMillan, D. W. C. Silyl radical activation of alkyl halides in metallaphotoredox catalysis: a unique pathway for cross-electrophile coupling. J. Am. Chem. Soc. 138, 8084–8087 (2016).
Harwood, S. J. et al. Modular terpene synthesis enabled by mild electrochemical couplings. Science 375, 745–752 (2022).
Le, C. C. et al. A general small-scale reactor to enable standardization and acceleration of photocatalytic reactions. ACS Cent. Sci. 3, 647–653 (2017).
Hansen, E. C. et al. New ligands for nickel catalysis from diverse pharmaceutical heterocycle libraries. Nat. Chem. 8, 1126–1130 (2016).
Choi, J. & Fu, G. C. Transition metal-catalyzed alkyl–alkyl bond formation: another dimension in cross-coupling chemistry. Science 356, eaaf7230 (2017).
Johnston, C. P., Smith, R. T., Allmendinger, S. & MacMillan, D. W. C. Metallaphotoredox-catalysed sp3–sp3 cross-coupling of carboxylic acids with alkyl halides. Nature 536, 322–325 (2016).
Sakai, H. A. & Macmillan, D. W. C. Nontraditional fragment couplings of alcohols and carboxylic acids: C(sp3)–C(sp3) cross-coupling via radical sorting. J. Am. Chem. Soc. 144, 6185–6192 (2022).
Smith, R. T. et al. Metallaphotoredox-catalyzed cross-electrophile Csp3–Csp3 coupling of aliphatic bromides. J. Am. Chem. Soc. 140, 17433–17438 (2018).
Dawson, G. A., Spielvogel, E. H. & Diao, T. Nickel-catalyzed radical mechanisms: informing cross-coupling for synthesizing non-canonical biomolecules. Acc. Chem. Res. 56, 3640–3653 (2023).
Kranthikumar, R. Recent advances in C(sp3)–C(sp3) cross-coupling chemistry: a dominant performance of nickel catalysts. Organometallics 41, 667–679 (2022).
Liu, W., Lavagnino, M. N., Gould, C. A., Alcázar, J. & MacMillan, D. W. C. A biomimetic SH2 cross-coupling mechanism for quaternary sp3-carbon formation. Science 374, 1258–1263 (2021).
Gan, X. et al. Carbon quaternization of redox active esters and olefins by decarboxylative coupling. Science 384, 113–118 (2024).
Zhang, W. et al. Electrochemically driven cross-electrophile coupling of alkyl halides. Nature 604, 292–297 (2022).
Dinh, L. P. et al. Persistent organonickel complexes as general platforms for Csp2–Csp3 coupling reactions. Nat. Chem. https://doi.org/10.1038/s41557-024-01528-7 (2024).
Kitiachvili, K. D., Mindiola, D. J. & Hillhouse, G. L. Preparation of stable alkyl complexes of Ni(I) and their one-electron oxidation to Ni(II) complex cations. J. Am. Chem. Soc. 126, 10554–10555 (2004).
Wagner, C. L. & Diao, T. in Comprehensive Organometallic Chemistry IV (eds Parkin, G. et al.) 271–356 (Elsevier, 2022).
Csok, Z., Vechorkin, O., Harkins, S. B., Scopelliti, R. & Hu, X. Nickel complexes of a pincer NN2 ligand: multiple carbon–chloride activation of CH2Cl2 and CHCl3 leads to selective carbon–carbon bond formation. J. Am. Chem. Soc. 130, 8156–8157 (2008).
Anderson, T. J., Jones, G. D. & Vicic, D. A. Evidence for a NiI active species in the catalytic cross-coupling of alkyl electrophiles. J. Am. Chem. Soc. 126, 8100–8101 (2004).
Jones, G. D. et al. Ligand redox effects in the synthesis, electronic structure, and reactivity of an alkyl–alkyl cross-coupling catalyst. J. Am. Chem. Soc. 128, 13175–13183 (2006).
Griego, L., Chae, J. B. & Mirica, L. M. A bulky 1,4,7-triazacyclononane and acetonitrile, a Goldilocks system for probing the role of NiIII and NiI centers in cross-coupling catalysis. Chem 10, 867–881 (2024).
Akana, M. E. et al. Computational methods enable the prediction of improved catalysts for nickel-catalyzed cross-electrophile coupling. J. Am. Chem. Soc. 146, 3043–3051 (2024).
Rein, J. et al. Unlocking the potential of high-throughput experimentation for electrochemistry with a standardized microscale reactor. ACS Cent. Sci. 7, 1347–1355 (2021).
Gatazka, M. R., McFee, E. C., Ng, C. H., Wearing, E. R. & Schindler, C. S. New strategies for the synthesis of 1- and 2-azetines and their applications as value-added building blocks. Org. Biomol. Chem. 20, 9052–9068 (2022).
Wang, Y. & Begley, T. P. Mechanistic studies on CysS—a vitamin B12-dependent radical SAM methyltransferase involved in the biosynthesis of the tert-butyl group of cystobactamid. J. Am. Chem. Soc. 142, 9944–9954 (2020).
Bour, J. R., Ferguson, D. M., McClain, E. J., Kampf, J. W. & Sanford, M. S. Connecting organometallic Ni(III) and Ni(IV): reactions of carbon-centered radicals with high-valent organonickel complexes. J. Am. Chem. Soc. 141, 8914–8920 (2019).
Chen, R. et al. Alcohol–alcohol cross-coupling enabled by SH2 radical sorting. Science 383, 1350–1357 (2024).
Qin, T. et al. A general alkyl–alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents. Science 352, 801–805 (2016).
Lin, Q., Spielvogel, E. H. & Diao, T. Carbon-centered radical capture at nickel(II) complexes: spectroscopic evidence, rates, and selectivity. Chem 9, 1295–1308 (2023).
Watson, M. B., Rath, N. P. & Mirica, L. M. Oxidative C–C bond formation reactivity of organometallic Ni(II), Ni(III), and Ni(IV) complexes. J. Am. Chem. Soc. 139, 35–38 (2017).
Lin, Q., Dawson, G. & Diao, T. Experimental electrochemical potentials of nickel complexes. Synlett 32, 1606–1620 (2021).
Acknowledgements
This work was supported by the National Institutes of Health (NIH R35 GM138373) and a Camille and Henry Dreyfus Teacher Scholar Award to C.S.S. V.A. thanks the TÜBİTAK - BİDEB (2214-A International Research Fellowship Program for PhD Students) for a scholarship. We thank L. Lewis for assistance with EPR spectroscopic measurements.
Author information
Authors and Affiliations
Contributions
S.A., D.K. and C.S.S. conceived the work and designed the experiments. S.W. and V.A. contributed equally. S.A., S.W., V.A., H.F.S. and M.M. performed all experiments and collected all data. S.A., S.W., V.A., H.F.S. and M.M. synthesized all substrates. H.F.S. performed parallel electrolysis reactions. C.E.M. performed collection and refinement of crystallographic data. M.M. performed computational studies. All authors analysed the data. S.A. and C.S.S. wrote the paper and all authors provided revisions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Kaid Harper and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains Supplementary Information; for details, see Table of Contents.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Al Zubaydi, S., Waske, S., Akyildiz, V. et al. Reductive alkyl–alkyl coupling from isolable nickel–alkyl complexes. Nature 634, 585–591 (2024). https://doi.org/10.1038/s41586-024-07987-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07987-9