Abstract
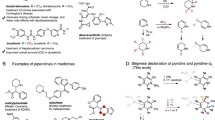
The hydrogen isotopes deuterium (D) and tritium (T) have become essential tools in chemistry, biology and medicine1. Beyond their widespread use in spectroscopy, mass spectrometry and mechanistic and pharmacokinetic studies, there has been considerable interest in incorporating deuterium into drug molecules1. Deutetrabenazine, a deuterated drug that is promising for the treatment of Huntington’s disease2, was recently approved by the United States’ Food and Drug Administration. The deuterium kinetic isotope effect, which compares the rate of a chemical reaction for a compound with that for its deuterated counterpart, can be substantial1,3,4. The strategic replacement of hydrogen with deuterium can affect both the rate of metabolism and the distribution of metabolites for a compound5, improving the efficacy and safety of a drug. The pharmacokinetics of a deuterated compound depends on the location(s) of deuterium. Although methods are available for deuterium incorporation at both early and late stages of the synthesis of a drug6,7, these processes are often unselective and the stereoisotopic purity can be difficult to measure7,8. Here we describe the preparation of stereoselectively deuterated building blocks for pharmaceutical research. As a proof of concept, we demonstrate a four-step conversion of benzene to cyclohexene with varying degrees of deuterium incorporation, via binding to a tungsten complex. Using different combinations of deuterated and proteated acid and hydride reagents, the deuterated positions on the cyclohexene ring can be controlled precisely. In total, 52 unique stereoisotopomers of cyclohexene are available, in the form of ten different isotopologues. This concept can be extended to prepare discrete stereoisotopomers of functionalized cyclohexenes. Such systematic methods for the preparation of pharmacologically active compounds as discrete stereoisotopomers could improve the pharmacological and toxicological properties of drugs and provide mechanistic information related to their distribution and metabolism in the body.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are available in the main text and Supplementary Information, including NMR spectra, experimental details, crystallographic information, DFT calculations, rotational spectroscopy and HRMS data. Supplementary crystallographic data for this paper (4, 7, 9 (X-ray) and 45 (neutron)) can be obtained from the Cambridge Crystallographic Data Centre at www.ccdc.cam.ac.uk/structures (CCDC 1885723-1885725 and 1972890).
References
Gant, T. G. Using deuterium in drug discovery: leaving the label in the drug. J. Med. Chem. 57, 3595–3611 (2014).
Dean, M. & Sung, V. W. Review of deutetrabenazine: a novel treatment for chorea asociated with Huntington’s disease. Drug Des. Devel. Ther. 12, 313–319 (2018).
Thibblin, A. & Ahlberg, P. Reaction branching and extreme kinetic isotope effects in the study of reaction mechanisms. Chem. Soc. Rev. 18, 209–224 (1989).
Thibblin, A. Unusually large kinetic deuterium isotope effects on oxidation reactions. 1. the mechanism of hydroxide-catalysed permanganate oxidation of PhCD(CF3)OH and PhCD(CH3)OH in water. J. Phys. Org. Chem. 8, 186–190 (1995).
Nelson, S. D. & Trager, W. F. The use of deuterium isotope effects to probe the active site properties, mechanism, of cyctochrome P450-catalyzed reactions, and mechanisms of metabolically dependent toxicity. Drug Metab. Dispos. 31, 1481–1497 (2003).
Loh, Y. Y. et al. Photoredox-catalyzed deuteration and tritiation of pharmaceutical compounds. Science 358, 1182–1187 (2017).
Pony Yu, R., Hesk, D., Rivera, N., Pelczer, I. & Chirik, P. J. Iron-catalysed tritiation of pharmaceuticals. Nature 529, 195–199 (2016).
Baldwin, J. E., Kiemle, D. J. & Kostikov, A. P. Quantitative analyses of stereoisomeric 3,4-d 2-cyclohexenes in the presence of 3,6-d 2-cyclohexenes. J. Org. Chem. 74, 3866–3874 (2009).
Eisen, M. S. & Marks, T. J. Supported organoactinide complexes as heterogeneous catalysts. A kinetic and mechanistic study of facile arene hydrogenation. J. Am. Chem. Soc. 114, 10358–10368 (1992).
Jones, R. A. & Seeberger, M. H. Synthesis of polymer-supported transition metal catalysts via phosphido linkages: heterogeneous catalysts for the hydrogenation of aromatic compounds under mild conditions. J. Chem. Soc. Chem. Commun. 373–374 (1985).
Joannou, M. V., Bezdek, M. J. & Chirik, P. J. Pyridine(diimine) molybdenum-catalyzed hydrogenation of arenes and hindered olefins: insights into precatalyst activation and deactivation pathways. ACS Catal. 8, 5276–5285 (2018).
Harman, W. D. & Taube, H. The selective hydrogenation of benzene to cyclohexene on pentaammineosmium(II). J. Am. Chem. Soc. 110, 7906–7907 (1988).
Liebov, B. K. & Harman, W. D. Group 6 dihapto-coordinate dearomatization agents for organic synthesis. Chem. Rev. 117, 13721–13755 (2017).
Welch, K. D. et al. Large-scale syntheses of several synthons to the dearomatization agent {TpW(NO)(PMe3)} and convenient spectroscopic tools for product analysis. Organometallics 26, 2791–2794 (2007).
Lankenau, A. W. et al. Enantioenrichment of a tungsten dearomatization agent utilizing chiral acids. J. Am. Chem. Soc. 137, 3649–3655 (2015).
Harrison, D. P. et al. Hyperdistorted tungsten allyl complexes and their stereoselective deprotonation to form dihapto-coordinated dienes. Organometallics 30, 2587–2597 (2011).
Lis, E. C. et al. The uncommon reactivity of dihapto-coordinated nitrile, ketone, and alkene ligands when bound to a powerful π-base. Organometallics 25, 5051–5058 (2006).
Arashiba, K. et al. Electrophilic O-methylation of a terminal nitrosyl ligand attained by an early−late heterobimetallic effect. Organometallics 25, 560–562 (2006).
Jamison, C. J. Isotope effects on chemical shifts and coupling constants. eMagRes https://doi.org/10.1002/9780470034590.emrstm0251 (2007).
Pérez, C. et al. Broadband Fourier transform rotational spectroscopy for structure determination: the water heptamer. Chem. Phys. Lett. 571, 1–15 (2013).
Sharp, W. B., Legzdins, P. & Patrick, B. O. O-Protonation of a terminal nitrosyl group to form an η1-hydroxylimido ligand. J. Am. Chem. Soc. 123, 8143–8144 (2001).
Llamazares, A., Schmalle, H. W. & Berke, H. Ligand-assisted heterolytic activation of hydrogen and silanes mediated by nitrosyl rhenium complexes. Organometallics 20, 5277–5288 (2001).
Leong, V. S. & Cooper, N. J. Electrophilic activation of benzene in [Cr(η 4–C6H6)(CO)3]2−. J. Am. Chem. Soc. 110, 2644–2646 (1988).
Thompson, R. L., Lee, S., Rheingold, A. L. & Cooper, N. J. Reductive activation of the coordinated benzene in manganese complex [Mn(η 6-C6H6)(CO)3]+: synthesis and characterization of the η 4-naphthalene complex PPN[Mn(η 4–C10H8)(CO)3]. Organometallics 10, 1657–1659 (1991).
Ungureanu, I.; Klotz, P.; Mann, A. Phenylaziridine as a masked 1,3 dipole in reactions with nonactivated alkenes. Angew. Chem. 39, 4615–4617 (1991).
Sarkar, N., Banerjee, A. & Nelson, S. G. [4 + 2] Cycloadditions of N-alkenyl iminium ions: structurally complex heterocycles from a three-component Diels−Alder reaction sequence. J. Am. Chem. Soc. 130, 9222–9223 (2008).
Shim, S. C., Doh, C. H., Kim, T. J., Lee, H. K. & Kim, K. D. A new and convenient synthesis of N-substituted perhydroazepines from adipaldehyde and primary amines with tetracarbonylhydridoferrate, HFe(CO)4 −, as a selective reducing agent. J. Heterocycl. Chem. 25, 1383–1385 (1988).
Wilson, K. B. et al. Sequential tandem addition to a tungsten–trifluorotoluene complex: a versatile method for the preparation of highly functionalized trifluoromethylated cyclohexenes. J. Am. Chem. Soc. 139, 11401–11412 (2017).
Murray, R. W., Singh, M., Williams, B. L. & Moncrieff, H. M. Diastereoselectivity in the epoxidation of substituted cyclohexenes by dimethyldioxirane. J. Org. Chem. 61, 1830–1841 (1996).
Leiris, S., Lucas, M., Dupuy d’Angeac, A. & Morère, A. Synthesis and biological evaluation of cyclic nitrogen mustards based on carnitine framework. Eur. J. Med. Chem. 45, 4140–4148 (2010).
Wilson, K. B. et al. Highly functionalized cyclohexenes derived from benzene: sequential tandem addition reactions promoted by tungsten. J. Org. Chem. 84, 6094–6116 (2019).
Acknowledgements
We acknowledge the assistance of E. Ashcraft in collecting HRMS data. The work was funded by the National Institutes of Health (1R01GM132205-01) and the University of Virginia. The single-crystal neutron diffraction experiment performed on TOPAZ using resources at the Spallation Neutron Source, a DOE Office of Science User Facility operated by the Oak Ridge National Laboratory, under contract number DE-AC05-00OR22725 with UT-Battelle, LLC.
Author information
Authors and Affiliations
Contributions
W.D.H., J.A.S. and K.B.W. conceived the project. K.D.W., W.D.H. and J.A.S. designed the experiments. J.A.S. and K.S.W. prepared the samples and collected NMR and HRMS data. D.A.D. carried out X-ray molecular structure determinations. B.H.P., P.J.K. and R.E.S. conceived and ran the rotational spectroscopy experiments. E.K.P. and K.S.W. carried out DFT calculations. J.A.S. and W.D.H. wrote the manuscript. X.W. collected and processed neutron diffraction data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks David Hesk and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains Supplementary Materials A-N, which includes Supplementary Figures S1-S15 and Supplementary Tables S1-S4.
Rights and permissions
About this article
Cite this article
Smith, J.A., Wilson, K.B., Sonstrom, R.E. et al. Preparation of cyclohexene isotopologues and stereoisotopomers from benzene. Nature 581, 288–293 (2020). https://doi.org/10.1038/s41586-020-2268-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2268-y
This article is cited by
-
Designing chemical systems for precision deuteration of medicinal building blocks
Nature Communications (2024)
-
Ambient hydrogenation of solid aromatics enabled by a high entropy alloy nanocatalyst
Nature Communications (2024)
-
Electrocatalytic reductive deuteration of arenes and heteroarenes
Nature (2024)
-
The double protonation of dihapto-coordinated benzene complexes enables dearomatization using aromatic nucleophiles
Nature Communications (2023)
-
Highly selective single and multiple deuteration of unactivated C(sp3)-H bonds
Nature Communications (2022)