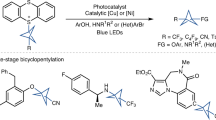
Abstract
Multicomponent reactions are relied on in both academic and industrial synthetic organic chemistry owing to their step- and atom-economy advantages over traditional synthetic sequences1. Recently, bicyclo[1.1.1]pentane (BCP) motifs have become valuable as pharmaceutical bioisosteres of benzene rings, and in particular 1,3-disubstituted BCP moieties have become widely adopted in medicinal chemistry as para-phenyl ring replacements2. These structures are often generated from [1.1.1]propellane via opening of the internal C–C bond through the addition of either radicals or metal-based nucleophiles3,4,5,6,7,8,9,10,11,12,13. The resulting propellane-addition adducts are then transformed to the requisite polysubstituted BCP compounds via a range of synthetic sequences that traditionally involve multiple chemical steps. Although this approach has been effective so far, a multicomponent reaction that enables single-step access to complex and diverse polysubstituted drug-like BCP products would be more time efficient compared to current stepwise approaches. Here we report a one-step three-component radical coupling of [1.1.1]propellane to afford diverse functionalized bicyclopentanes using various radical precursors and heteroatom nucleophiles via a metallaphotoredox catalysis protocol. This copper-mediated reaction operates on short timescales (five minutes to one hour) across multiple (more than ten) nucleophile classes and can accommodate a diverse array of radical precursors, including those that generate alkyl, α-acyl, trifluoromethyl and sulfonyl radicals. This method has been used to rapidly prepare BCP analogues of known pharmaceuticals, one of which is substantially more metabolically stable than its commercial progenitor.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information.
References
Dömling, A., Wang, W. & Wang, K. Chemistry and biology of multicomponent reactions. Chem. Rev. 112, 3083–3135 (2012).
Mykhailiuk, P. K. Saturated bioisosteres of benzene: where to go next? Org. Biomol. Chem. 17, 2839–2849 (2019).
Kanazawa, J. & Uchiyama, M. Recent advances in the synthetic chemistry of bicyclo[1.1.1]pentane. Synlett 30, 1–11 (2019).
Kanazawa, J., Maeda, K. & Uchiyama, M. Radical multicomponent carboamination of [1.1.1]propellane. J. Am. Chem. Soc. 139, 17791–17794 (2017).
Nugent, J. et al. A general route to bicyclo[1.1.1]pentanes through photoredox catalysis. ACS Catal. 9, 9568–9574 (2019).
Kondo, M. et al. Silaboration of [1.1.1]propellane to provide a storable feedstock for bicyclo[1.1.1]pentane derivatives. Angew. Chem. Int. Ed. 59, 1970 (2020).
Kaszynski, P. & Michl, J. A practical photochemical synthesis of bicyclo[1.1.1]pentane-1,3-dicarboxylic acid. J. Org. Chem. 53, 4593–4594 (1988).
Caputo, D. F. J. et al. Synthesis and applications of highly functionalized 1-halo-3-substituted bicyclo[1.1.1]pentanes. Chem. Sci. 9, 5295–5300 (2018).
Trongsiriwat, N. et al. Reactions of 2-aryl-1,3-dithianes and [1.1.1]propellane. Angew. Chem. Int. Ed. 58, 13416–13420 (2019).
Gianatassio, R. et al. Strain-release amination. Science 351, 241–246 (2016).
Makarov, I. S., Brocklehurst, C. E., Karaghiosoff, K., Koch, G. & Knochel, P. Synthesis of bicyclo[1.1.1]pentane bioisosteres of internal alkynes and para-disubstituted benzenes from [1.1.1]propellane. Angew. Chem. Int. Ed. 56, 12774–12777 (2017).
Hughes, J. M. E., Scarlata, D. A., Chen, A. C.-Y., Burch, J. D. & Gleason, J. L. Aminoalkylation of [1.1.1]propellane enables direct access to high-value 3-alkylbicyclo[1.1.1]pentan-1-amines. Org. Lett. 21, 6800–6804 (2019).
Wiberg, K. B. & Waddell, S. T. Reactions of [1.1.1]propellane. J. Am. Chem. Soc. 112, 2194–2216 (1990).
Stepan, A. F. et al. Application of the bicyclo[1.1.1]pentane motif as a nonclassical phenyl ring bioisostere in the design of a potent and orally active γ-secretase inhibitor. J. Med. Chem. 55, 3414–3424 (2012).
Measom, N. D. et al. Investigation of a bicyclo[1.1.1]pentane as a phenyl replacement within an LpPLA2 inhibitor. ACS Med. Chem. Lett. 8, 43–48 (2017).
Fischer, C. et al. Novel tricyclic compounds as inhibitors of mutant IDH enzymes. International patent WO/2016/089830 A1 (2016).
Sidrauski, C. et al. Modulators of the integrated stress pathway. International patent WO/2017/193030 A1 (2017).
Kaszynski, P., McMurdie, N. D. & Michl, J. Synthesis of doubly bridgehead substituted bicyclo[1.1.1]pentanes. Radical transformations of bridgehead halides and carboxylic acids. J. Org. Chem. 56, 307–316 (1991).
Twilton, J., Le, C., Zhang, P., Shaw, M. H., Evans, R. W. & MacMillan, D. W. C. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 1, 0052 (2017).
Kalyani, D., McMurtrey, K. B., Neufeldt, S. R. & Sanford, M. S. Room-temperature C–H arylation: merger of Pd-catalyzed C–H functionalization and visible-light photocatalysis. J. Am. Chem. Soc. 133, 18566–18569 (2011).
Primer, D. N. & Molander, G. A. Enabling the cross-coupling of tertiary organoboron nucleophiles through radical-mediated alkyl transfer. J. Am. Chem. Soc. 139, 9847–9850 (2017).
Le, C., Chen, T. Q., Liang, T., Zhang, P. & MacMillan, D. W. C. A radical approach to the copper oxidative addition problem: trifluoromethylation of bromoarenes. Science 360, 1010–1014 (2018).
Mao, R., Frey, A., Balon, J. & Hu, X. Decarboxylative C(sp 3)–N cross-coupling via synergetic photoredox and copper catalysis. Nat. Catal. 1, 120–126 (2018).
Zhao, W., Wurz, R. P., Peters, J. C. & Fu, G. C. Photoinduced, copper-catalyzed decarboxylative C–N coupling to generate protected amines: an alternative to the Curtius rearrangement. J. Am. Chem. Soc. 139, 12153–12156 (2017).
Liang, Y., Zhang, X. & MacMillan, D. W. C. Decarboxylative sp 3 C–N coupling via dual copper and photoredox catalysis. Nature 559, 83–88 (2018).
Banks, J. T., Ingold, K. U., Della, E. W. & Walton, J. C. Bicyclo[1.1.1]pent-1-yl: a tertiary radical with enhanced reactivity. Tetrahedr. Lett. 37, 8059–8060 (1996).
Dixon, I. M. et al. A family of luminescent coordination compounds: iridium(III) polyimine complexes. Chem. Soc. Rev. 29, 385–391 (2000).
Nacsa, E. D. & MacMillan, D. W. C. Spin-center shift-enabled direct enantioselective α-benzylation of aldehydes with alcohols. J. Am. Chem. Soc. 140, 3322–3330 (2018).
Minisci, F., Vismara, E., Fontana, F. & Barbosa, M. C. N. A new general method of homolytic alkylation of protonated heteroaromatic bases by carboxylic acids and iodosobenzene diacetate. Tetrahedr. Lett. 30, 4569–4572 (1989).
DiMucci, I. M. et al. The myth of d 8 copper(III). J. Am. Chem. Soc. 141, 18508–18520 (2019).
Walton, J. C. Bridgehead radicals. Chem. Soc. Rev. 21, 105–112 (1992).
Fiorentino, M., Testaferri, L., Tiecco, M. & Troisi, L. Structural effects on the reactivity of carbon radicals in homolytic aromatic substitution. Part 4. The nucleophilicity of bridgehead radicals. J. Chem. Soc. Perkin Trans. 2 2, 87–93 (1977).
Della, E. W., Cotsaris, E., Hine, P. T. & Pigou, P. E. 13C Spectral parameters of some polycyclic hydrocarbons. II. Bicyclo[3,1,1]heptane, tricyclo[3,1,1,03,6]heptane, tricyclo[3,3,0,02,6]octane and bicyclo[1,1,1]pentane. Aust. J. Chem. 34, 913–916 (1981).
Silvi, M. & Aggarwal, V. K. Radical addition to strained σ-bonds enables the stereocontrolled synthesis of cyclobutyl boronic esters. J. Am. Chem. Soc. 141, 9511–9515 (2019).
Acknowledgements
Research reported in this publication was supported by the NIH National Institute of General Medical Sciences (1R35GM134897-01) and gifts from Merck, Bristol-Myers Squibb, Eli Lilly and Janssen Research and Development LLC. We acknowledge Y. Liang for discussions.
Author information
Authors and Affiliations
Contributions
X.Z., R.T.S., C.L. and S.J.M. performed and analysed the experiments. X.Z., R.T.S., C.L. and D.W.C.M. designed the experiments. S.J.M., B.T.S. and N.I.C. provided intellectual contributions. R.T.S., X.Z. and D.W.C.M. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, X., Smith, R.T., Le, C. et al. Copper-mediated synthesis of drug-like bicyclopentanes. Nature 580, 220–226 (2020). https://doi.org/10.1038/s41586-020-2060-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2060-z
This article is cited by
-
Difunctionalization of bicyclo[1.1.0]butanes enabled by merging C−C cleavage and ruthenium-catalysed remote C−H activation
Nature Synthesis (2025)
-
Synthesis of bicyclo[3.1.1]heptanes, meta-substituted arene isosteres, from [3.1.1]propellane
Nature Protocols (2025)
-
Enantioselective synthesis of 2-substituted bicyclo[1.1.1]pentanes via sequential asymmetric imine addition of bicyclo[1.1.0]butanes and skeletal editing
Nature Chemistry (2025)
-
Synthesis of tertiary alkyl amines via photoinduced copper-catalysed nucleophilic substitution
Nature Chemistry (2025)
-
Modular access to saturated bioisosteres of anilines via photoelectrochemical decarboxylative C(sp3)–N coupling
Nature Communications (2025)