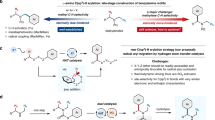
Abstract
Methods for selective C–H bond functionalization have provided chemists with versatile and powerful toolboxes for synthesis, such as the late-stage modification of a lead compound without the need for lengthy de novo synthesis1,2,3,4,5. Cleavage of an sp3 C–H bond via hydrogen atom transfer (HAT) is particularly useful, given the large number of available HAT acceptors and the diversity of reaction pathways available to the resulting radical intermediate6,7,8,9,10,11,12,13,14,15,16,17. Site-selectivity, however, remains a formidable challenge, especially among sp3 C–H bonds with comparable properties. If the intermediate radical could be further trapped enantioselectively, this should enable highly site- and enantioselective functionalization of C–H bonds. Here we report a copper (Cu)-catalysed site- and enantioselective allylic C–H cyanation of complex alkenes, in which a Cu(ii)-bound nitrogen (N)-centred radical plays the key role in achieving precise site-specific HAT. This method is shown to be effective for a diverse collection of alkene-containing molecules, including sterically demanding structures and complex natural products and pharmaceuticals.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information. Metrical parameters for the structure of reagents 2c, 2d and products 10d, 17c and S8 (see Supplementary Information) are available free of charge from the Cambridge Crystallographic Data Centre (https://www.ccdc.cam.ac.uk/) under reference numbers CCDC 1945032, CCDC 1945034, CCDC 1945035, CCDC 1945033 and CCDC 1945036, respectively.
References
Cernak, T., Dykstra, K. D., Tyagarajan, S., Vachal, P. & Krska, S. W. The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 45, 546–576 (2016).
Davies, H. M. L. & Morton, D. Guiding principles for site-selective and stereoselective intermolecular C–H functionalization by donor/acceptor rhodium carbenes. Chem. Soc. Rev. 40, 1857–1869 (2011).
Zhang, F.-L., Hong, K., Li, T.-J., Park, H. & Yu, J.-Q. Functionalization of C(sp 3)-H bonds using a transient directing group. Science 351, 252–256 (2016).
McNally, A., Haffemayer, B., Collins, B. S. L. & Gaunt, M. J. Palladium-catalysed C–H activation of aliphatic amines to give strained nitrogen heterocycles. Nature 510, 129–133 (2014).
Prier, C. K., Zhang, R. K., Buller, A. R., Brinkmann-Chen, S. & Arnold, F. H. Enantioselective, intermolecular benzylic C–H amination catalyzed by an engineered iron-haem enzyme. Nat. Chem. 9, 629–634 (2017).
Sharma, A. & Hartwig, J. F. Metal-catalyzed azidation of tertiary C–H bonds suitable for late-stage functionalization. Nature 517, 600–604 (2015).
Le, C., Liang, Y., Evans, R. W., Li, X. & MacMillan, D. W. C. Selective sp 3 C–H alkylation via polarity-match-based cross-coupling. Nature 547, 79–83 (2017).
Chen, M. S. & White, M. C. Combined effects on selectivity in Fe-catalyzed methylene oxidation. Science 327, 566–571 (2010).
Horn, E. J. et al. Scalable and sustainable electrochemical allylic C–H oxidation. Nature 533, 77–81 (2016).
Liu, W. & Groves, J. T. Manganese catalyzed C–H halogenation. Acc. Chem. Res. 48, 1727–1735 (2015).
Che, C.-M., Lo, V. K.-Y., Zhou, C.-Y. & Huang, J.-S. Selective functionalisation of saturated C−H bonds with metalloporphyrin catalysts. Chem. Soc. Rev. 40, 1950–1975 (2011).
Jeffrey, J. L., Terrett, J. A. & MacMillan, D. W. C. O–H hydrogen bonding promotes H-atom transfer from a C–H bonds for α-alkylation of alcohols. Science 349, 1532–1536 (2015).
Schmidt, V. A., Quinn, R. K., Brusoe, A. T. & Alexanian, E. J. Site-selective aliphatic C–H bromination using N-bromoamides and visible light. J. Am. Chem. Soc. 136, 14389–14392 (2014).
Milan, M., Salamone, M., Costas, M. & Bietti, M. The quest for selectivity in hydrogen atom transfer based aliphatic C–H bond oxygenation. Acc. Chem. Res. 51, 1984–1995 (2018).
Carestia, A. M., Ravelli, D. & Alexanian, E. J. Reagent-dictated site selectivity in intermolecular aliphatic C–H functionalizations using nitrogen-centered radicals. Chem. Sci. 9, 5360–5365 (2018).
Shields, B. J. & Doyle, A. G. Direct C(sp 3)–H cross coupling enabled by catalytic generation of chlorine radicals. J. Am. Chem. Soc. 138, 12719–12722 (2016).
Milan, M., Bietti, M. & Costas, M. Highly enantioselective oxidation of nonactivated aliphatic C–H bonds with hydrogen peroxide catalyzed by manganese complexes. ACS Cent. Sci. 3, 196–204 (2017).
Covell, D. J. & White, M. C. A chiral Lewis acid strategy for the enantioselective allylic C–H oxidation. Angew. Chem. Int. Ed. 47, 6448–6451 (2008).
Bayeh, L., Le, P. Q. & Tambar, U. K. Catalytic allylic oxidation of internal alkenes to a multifunctional chiral building block. Nature 547, 196–200 (2017).
Kharasch, M. S. & Sosnovsky, G. The reactions of t-butyl perbenzoate and olefins—a stereospecific reaction. J. Am. Chem. Soc. 80, 756 (1958).
Eames, J. & Watkinson, M. Catalytic allylic oxidation of alkenes using an asymmetric Kharasch–Sosnovsky reaction. Angew. Chem. Int. Ed. 40, 3567–3571 (2001).
Andrus, M. B. & Zhou, Z. Highly enantioselective copper-bisoxazoline-catalyzed allylic oxidation of cyclic olefins with tert-butyl p-nitroperbenzoate. J. Am. Chem. Soc. 124, 8806–8807 (2002).
Wang, F. et al. Enantioselective copper-catalyzed intermolecular cyanotrifluoromethylation of alkenes via radical process. J. Am. Chem. Soc. 138, 15547–15550 (2016).
Zhang, W. et al. Enantioselective cyanation of benzylic C–H bonds via copper-catalyzed radical relay. Science 353, 1014–1018 (2016).
Czaplyski, W. L., Na, C. G. & Alexanian, E. J. C–H xanthylation: a synthetic platform for alkane functionalization. J. Am. Chem. Soc. 138, 13854–13857 (2016).
Kärkäs, M. D. Photochemical generation of nitrogen-centered amidyl, hydrazonyl, and imidyl radicals: methodology development and catalytic applicants. ACS Catal. 7, 4999–5022 (2017).
Artaryan, A. et al. Aliphatic C−H bond iodination by a N-iodoamide and isolation of an elusive N-amidyl radical. J. Org. Chem. 82, 7093–7100 (2017).
Palucki, M. et al. The mechanistic basis for electronic effect on enantioselectivity in the (salen)Mn(ii)-catalyzed epoxidation reaction. J. Am. Chem. Soc. 120, 948–954 (1998).
Barham, J. P., John, M. P. & Murphy, J. A. Contra-thermodynamic hydrogen atom abstraction in the selective C−H functionalization of trialkylamine N-CH3 groups. J. Am. Chem. Soc. 138, 15482–15487 (2016).
Peters, J.-U. 11 years of cyanopyrrolidines as DPP-IV inhibitors. Curr. Top. Med. Chem. 7, 579–595 (2007).
Liao, K. et al. Site-selective and stereoselective functionalization of non-activated tertiary C–H bonds. Nature 551, 609–613 (2017).
Chen, M. S. & White, M. C. A predictably selective aliphatic C–H oxidation reaction for complex molecule synthesis. Science 318, 783–787 (2007).
Fleming, F. F., Yao, L., Ravikumar, P. C., Funk, L. & Shook, B. C. Nitrile-containing pharmaceuticals: efficacious roles of the nitrile pharmacophore. J. Med. Chem. 53, 7902–7917 (2010).
Acknowledgements
Financial support was provided by the National Basic Research Program of China (grant number 973-2015CB856600), the National Natural Science Foundation of China (grant numbers 21532009, 21821002, 21790330 and 21761142010), the Science and Technology Commission of Shanghai Municipality (grant numbers 17XD1404500, 17QA1405200 and 17JC1401200), the Strategic Priority Research Program (grant number XDB20000000), the Key Research Program of Frontier Science (grant number QYZDJSSW-SLH055) and the Youth Innovation Promotion Association (grant number 2018292) of the Chinese Academy of Sciences, and the Research Grants Council of Hong Kong (grant numbers HKUST 16304416 and 16304017). J.L. thanks Y. Zhang for performing the electron paramagnetic resonance manipulation and analysis and X. Wan for HPLC analysis. G.L. thanks H. Guan (University of Cincinnati) and S. Stahl (University of Wisconsin-Madison) for discussions.
Author information
Authors and Affiliations
Contributions
J.L., L.W., W.Z. and G.L. conceived the work and designed the experiments. J.L. performed most of the laboratory experiments (with help from L.W. and W.Z.). Z.Z. and Z.L. conducted density functional theory calculations. J.L., P.C., Z.L. and G.L. analysed the data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Peer review information Nature thanks John Murphy, Peter Richard Schreiner and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Supplementary information
Supplementary Information
Supporting materials, including experimental procedure, mechanistic study, DFT calculation, and compound characterization.
Rights and permissions
About this article
Cite this article
Li, J., Zhang, Z., Wu, L. et al. Site-specific allylic C–H bond functionalization with a copper-bound N-centred radical. Nature 574, 516–521 (2019). https://doi.org/10.1038/s41586-019-1655-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1655-8
This article is cited by
-
Anti-Markovnikov hydroallylation reaction of alkenes via scandium-catalyzed allylic C‒H activation
Nature Communications (2025)
-
Site- and enantioselective allylic and propargylic C–H oxidation enabled by copper-based biomimetic catalysis
Nature Catalysis (2025)
-
Nickel-catalysed enantioselective alkene dicarbofunctionalization enabled by photochemical aliphatic C–H bond activation
Nature Catalysis (2024)
-
Site-selective arene C–H amination with iron-aminyl radical
Nature Catalysis (2024)
-
Cooperative Cu/azodiformate system-catalyzed allylic C–H amination of unactivated internal alkenes directed by aminoquinoline
Nature Communications (2024)