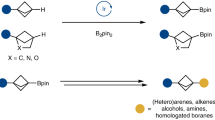
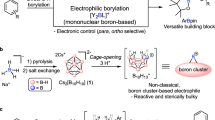
Abstract
Organoboron reagents are important synthetic intermediates that have a key role in the construction of natural products, pharmaceuticals and organic materials1. The discovery of simpler, milder and more efficient approaches to organoborons can open additional routes to diverse substances2,3,4,5. Here we show a general method for the directed C–H borylation of arenes and heteroarenes without the use of metal catalysts. C7- and C4-borylated indoles are produced by a mild approach that is compatible with a broad range of functional groups. The mechanism, which is established by density functional theory calculations, involves BBr3 acting as both a reagent and a catalyst. The potential utility of this strategy is highlighted by the downstream transformation of the formed boron species into natural products and drug scaffolds.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information. Additional data are available from the corresponding authors upon request. Metrical parameters for the structures of 1b, 9c, 28b, 28c and 1d are available free of charge from the Cambridge Crystallographic Data Centre (https://www. ccdc.cam.ac.uk/) under reference numbers CCDC 1910131, 1910132, 1910134, 1910135 and 1910137, respectively.
References
Suzuki, A. Cross-coupling reactions of organoboranes: an easy way to construct C–C bonds (Nobel Lecture). Angew. Chem. Int. Ed. 50, 6722–6737 (2011).
Cook, A. K., Schimler, S. D., Matzger, A. J. & Sanford, M. S. Catalyst-controlled selectivity in the C–H borylation of methane and ethane. Science 351, 1421–1424 (2016).
Smith, K. T. et al. Catalytic borylation of methane. Science 351, 1424–1427 (2016).
Fawcett, A. et al. Photoinduced decarboxylative borylation of carboxylic acids. Science 357, 283–286 (2017).
Li, C. et al. Decarboxylative borylation. Science 356, eaam7355 (2017).
Snieckus, V. Directed ortho metalation. Tertiary amide and O-carbamate directors in synthetic strategies for polysubstituted aromatics. Chem. Rev. 90, 879–933 (1990).
Murai, S. et al. Efficient catalytic addition of aromatic carbon–hydrogen bonds to olefins. Nature 366, 529–531 (1993).
Gensch, T., Hopkinson, M. N., Glorius, F. & Wencel-Delord, J. Mild metal-catalyzed C–H activation: examples and concepts. Chem. Soc. Rev. 45, 2900–2936 (2016).
Cho, J.-Y., Tse, M. K., Holmes, D., Maleczka, R. E., Jr & Smith, M. R., III. Remarkably selective iridium catalysts for the elaboration of aromatic C–H bonds. Science 295, 305–308 (2002).
Mkhalid, I. A. I., Barnard, J. H., Marder, T. B., Murphy, J. M. & Hartwig, J. F. C–H activation for the construction of C–B bonds. Chem. Rev. 110, 890–931 (2010).
Ros, A., Fernández, R. & Lassaletta, J. M. Functional group directed C–H borylation. Chem. Soc. Rev. 43, 3229–3243 (2014).
Letsinger, R. L. & MacLean, D. B. Organoboron compounds. XVI. Cooperative functional group effects in reactions of boronoarylbenzimidazoles. J. Am. Chem. Soc. 85, 2230–2236 (1963).
Davis, F. A. & Dewar, M. J. S. New heteroaromatic compounds. XXX. A derivative of 10, 9-borathiarophenanthrene. J. Am. Chem. Soc. 90, 3511–3515 (1968).
Ishida, N., Moriya, T., Goya, T. & Murakami, M. Synthesis of pyridine–borane complexes via electrophilic aromatic borylation. J. Org. Chem. 75, 8709–8712 (2010).
Berger, F. et al. Site-selective and versatile aromatic C–H functionalization by thianthrenation. Nature 567, 223–228 (2019).
Stuart, D. R. & Fagnou, K. The catalytic cross-coupling of unactivated arenes. Science 316, 1172–1175 (2007).
Sandtorv, A. H. Transition metal-catalysed C–H activation of indoles. Adv. Syn. Cat. 357, 2403–2435 (2015).
Légaré, M.-A., Courtemanche, M.-A., Rochette, É. & Fontaine, F.-G. Metal-free catalytic C–H bond activation and borylation of heteroarenes. Science 349, 513–516 (2015).
Toutov, A. A. et al. Silylation of C–H bonds in aromatic heterocycles by an Earth-abundant metal catalyst. Nature 518, 80–84 (2015).
Robbins, D. W., Boebel, T. A. & Hartwig, J. F. Iridium-catalyzed, silyl-directed borylation of nitrogen-containing heterocycles. J. Am. Chem. Soc. 132, 4068–4069 (2010).
Yang, Y., Qiu, X., Zhao, Y., Mu, Y. & Shi, Z. Palladium-catalyzed C–H arylation of indoles at the C7 position. J. Am. Chem. Soc. 138, 495–498 (2016).
Xu, L., Zhang, C., He, Y., Tan, L. & Ma, D. Rhodium-catalysed regioselective C7-functionalization of N-pivaloylindoles. Angew. Chem. Int. Ed. 55, 321–325 (2016).
Yang, Y., Gao, P., Zhao, Y. & Shi, Z. Regiocontrolled direct C–H arylation of indoles at the C4 and C5 positions. Angew. Chem. Int. Ed. 56, 3966–3971 (2017).
Qiu, X., Deng, H., Zhao, Y. & Shi, Z. Rhodium-catalyzed, P-directed selective C7 arylation of indoles. Sci. Adv. 4, eaau6468 (2018).
Qiu, X. et al. PIII-chelation-assisted indole C7-arylation, olefination, methylation, and acylation with carboxylic acids/anhydrides by rhodium catalysis. Angew. Chem. Int. Ed. 58, 1504–1508 (2019).
Wang, D., Xue, X.-S., Houk, K. N. & Shi, Z. Mild ring-opening 1,3-hydroborations of non-activated cyclopropanes. Angew. Chem. Int. Ed. 57, 16861–16865 (2018).
Wang, C. & Sperry, J. Iridium-catalyzed C–H borylation-based synthesis of natural indolequinones. J. Org. Chem. 77, 2584–2587 (2012).
Zhang, Y.-H. & Yu, J.-Q. Pd(II)-catalyzed hydroxylation of arenes with 1 atm of O2 or air. J. Am. Chem. Soc. 131, 14654–14655 (2009).
Barluenga, J., Tomás-Gamasa, M., Aznar, F. & Valdés, C. Metal-free carbon–carbon bond-forming reductive coupling between boronic acids and tosylhydrazones. Nat. Chem. 1, 494–499 (2009).
De Vries, T. S., Prokofjevs, A. & Vedejs, E. Cationic tricoordinate boron intermediates: borenium chemistry from the organic perspective. Chem. Rev. 112, 4246–4282 (2012).
Acknowledgements
We thank group members Z. Li, X. Han, H. Deng, Z. Zhu and J. Qian for reproducing the results in the project. We acknowledge the Chinese ‘Thousand Youth Talents Plan’, the National Natural Science Foundation of China (grant 21672097), and the ‘Innovation & Entrepreneurship Talents Plan’ of Jiangsu Province in China (to Z.S.), and the National Science Foundation of the USA (CHE-1764328 to K.N.H.) for financial support.
Author information
Authors and Affiliations
Contributions
Z.S. conceived the project and directed the research. K.N.H. and Y. Liang supervised the mechanistic study. Z.S. and K.N.H. wrote the paper. J.L., B.Z. and M.W. performed the experiments. X.C. and X.-S.X. performed the DFT calculations. L.J. assisted with operando infrared spectroscopy experiments. Y.Z. performed the crystallographic studies. Y.Y., Y.H., Y. Lu, J.Z. and W.-Y.S. discussed the results.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Peer review information Nature thanks Julia Rehbein and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Supplementary information
Supplementary Information
This file contains: 1. General information; 2. Synthesis of substrates; 3. Investigation of reaction conditions; 4. Experimental procedures and characterization of products; 5. Applications of metal-free directed C-H borylation strategy; 6. Mechanistic investigations; 7. Crystallographic data; 8. References; and 9. Copies of 1H, 13C and 19F NMR spectra.
Rights and permissions
About this article
Cite this article
Lv, J., Chen, X., Xue, XS. et al. Metal-free directed sp2-C–H borylation. Nature 575, 336–340 (2019). https://doi.org/10.1038/s41586-019-1640-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1640-2