Abstract
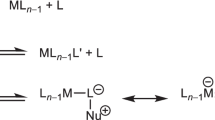
Transition-metal complexes are widely used in the physical and biological sciences. They have essential roles in catalysis, synthesis, materials science, photophysics and bioinorganic chemistry. Our understanding of transition-metal complexes originates from Alfred Werner’s realization that their three-dimensional shape influences their properties and reactivity1, and the intrinsic link between shape and electronic structure is now firmly underpinned by molecular-orbital theory2,3,4,5. Despite more than a century of advances in this field, the geometries of transition-metal complexes remain limited to a few well-understood examples. The archetypal geometries of six-coordinate transition metals are octahedral and trigonal prismatic, and although deviations from ideal bond angles and bond lengths are frequent6, alternative parent geometries are extremely rare7. The hexagonal planar coordination environment is known, but it is restricted to condensed metallic phases8, the hexagonal pores of coordination polymers9, or clusters that contain more than one transition metal in close proximity10,11. Such a geometry had been considered12,13 for [Ni(PtBu)6]; however, an analysis of the molecular orbitals suggested that this complex is best described as a 16-electron species with a trigonal planar geometry14. Here we report the isolation and structural characterization of a simple coordination complex in which six ligands form bonds with a central transition metal in a hexagonal planar arrangement. The structure contains a central palladium atom surrounded by three hydride and three magnesium-based ligands. This finding has the potential to introduce additional design principles for transition-metal complexes, with implications for several scientific fields.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Crystallographic models are available as .cif files from the Cambridge Crystallographic Data Centre (CCDC, https://www.ccdc.cam.ac.uk); CCDC numbers 1589323–1589324, 1909687–1909690 and 1946045). Neutron diffraction images can be obtained from A.J.E. The derived structure factors have been deposited with the CCDC, with CCDC number 1946045. Data associated with DFT calculations (.xyz coordinate file) along with NMR spectroscopic data (.mnova files) are available from a public repository at https://doi.org/10.14469/hpc/5985. Full details of the syntheses are provided in the Supplementary Information.
References
Werner, A. On the constitution and configuration of higher-order compounds. Nobel Lecture, 11 December 1913. The Nobel Prize https://www.nobelprize.org/prizes/chemistry/1913/werner/lecture (1913).
Hoffmann, R., Beier, B. F., Muetterties, E. L. & Rossi, A. R. Seven-coordination. A molecular orbital exploration of structure, stereochemistry, and reaction dynamics. Inorg. Chem. 16, 511–522 (1977).
Rossi, A. R. & Hoffmann, R. Transition metal pentacoordination. Inorg. Chem. 14, 365–374 (1975).
Gray, H. B. & Ballhausen, C. J. A molecular orbital theory for square planar metal complexes. J. Am. Chem. Soc. 85, 260–265 (1963).
Gray, H. B. Molecular orbital theory for transition metal complexes. J. Chem. Educ. 41, 2–12 (1964).
Alvarez, S. Distortion pathways of transition metal coordination polyhedra induced by chelating topology. Chem. Rev. 115, 13447–13483 (2015).
García-Monforte, M. A., Baya, M., Falvello, L. R., Martín, A. & Menjón, B. An organotransition-metal complex with pentagonal-pyramidal structure. Angew. Chem. 51, 8046–8049 (2012).
Yang, L.-M. et al. Two-dimensional Cu2Si monolayer with planar hexacoordinate copper and silicon bonding. J. Am. Chem. Soc. 137, 2757–2762 (2015).
Niu, Z., Ma, J.-G., Shi, W. & Cheng, P. Water molecule-driven reversible single-crystal to single-crystal transformation of a multi-metallic coordination polymer with controllable metal ion movement. Chem. Commun. 50, 1839–1841 (2014).
Tanabe, M. et al. Tetrapalladium complex with bridging germylene ligands. Structural change of the planar Pd4Ge3 core. J. Am. Chem. Soc. 133, 18598–18601 (2011).
Yamada, T., Mawatari, A., Tanabe, M., Osakada, K. & Tanase, T. Planar tetranuclear and dumbbell-shaped octanuclear palladium complexes with bridging silylene ligands. Angew. Chem. 48, 568–571 (2009).
Ahlrichs, R., Fenske, D., Oesen, H. & Schneider, U. Synthesis and structure of [Ni(PtBu)6] and [Ni5(PtBu)6(CO)5] and calculations on the electronic structure of [Ni(PtBu)6] and (PR)6, R = tBu,Me. Angew. Chem. 31, 323–326 (1992).
Hey-Hawkins, E., Pink, M., Oesen, H. & Fenske, D. Synthesen und charakterisierung von [Ni(tBuAs)6] und [Pd(tBuAs)6]. Z. Anorg. Allg. Chem. 622, 689–691 (1996).
Tang, H., Hoffman, D. M., Albright, T. A., Deng, H. & Hoffmann, R. [Ni(PtBu)6] and [Ni(SiH2)6] are isolobal, related to [In{Mn(CO)4}5]2−, and have 16-electron counts. Angew. Chem. 32, 1616–1618 (1993).
Kubas, G. J. Dihydrogen complexes as prototypes for the coordination chemistry of saturated molecules. Proc. Natl Acad. Sci. USA 104, 6901–6907 (2007).
Crabtree, R. H. A new oxidation state for Pd? Science 295, 288–289 (2002).
Chen, W., Shimada, S. & Tanaka, M. Synthesis and structure of formally hexavalent palladium complexes. Science 295, 308–310 (2002).
Aullón, G., Lledós, A. & Alvarez, S. Hexakis(silyl)palladium(vi) or palladium(ii) with η2-disilane ligands? Angew. Chem. 41, 1956–1959 (2002).
Butler, M. J. & Crimmin, M. R. Magnesium, zinc, aluminium and gallium hydride complexes of the transition metals. Chem. Commun. 53, 1348–1365 (2017).
Abdalla, J. A. B. et al. Coordination and activation of Al–H and Ga–H bonds. Chem. Eur. J. 20, 17624–17634 (2014).
Abdalla, J. A. B. et al. Structural snapshots of concerted double E–H bond activation at a transition metal centre. Nat. Chem. 9, 1256–1262 (2017).
Riddlestone, I. M. et al. Activation of H2 over the Ru–Zn bond in the transition metal–Lewis acid heterobimetallic species [Ru(IPr)2(CO)ZnEt]+. J. Am. Chem. Soc. 138, 11081–11084 (2016).
Sharninghausen, L. S. et al. The neutron diffraction structure of [Ir4(IMe)8H10]2+ polyhydride cluster: testing the computational hydride positional assignments. J. Organomet. Chem. 849–850, 17–21 (2017).
Pauling, L. Atomic radii and interatomic distances in metals. J. Am. Chem. Soc. 69, 542–553 (1947).
Pyykkö, P. & Atsumi, M. Molecular single-bond covalent radii for elements 1–118. Chem. Eur. J. 15, 186–197 (2009).
Mukherjee, D. & Okuda, J. Molecular magnesium hydrides. Angew. Chem. 57, 1458–1473 (2018).
Brookhart, M., Green, M. L. H. & Parkin, G. Agostic interactions in transition metal compounds. Proc. Natl Acad. Sci. USA 104, 6908–6914 (2007).
Shoshani, M. M., Liu, J. & Johnson, S. A. Mechanistic insight into H/D exchange by a pentanuclear Ni–H cluster and synthesis and characterization of structural analogues of potential intermediates. Organometallics 37, 116–126 (2018).
Kudo, K., Hidai, M. & Uchida, Y. Dihydride complexes of platinum(ii) and palladium(ii). J. Organomet. Chem. 56, 413–418 (1973).
Leviston, P. G. & Wallbridge, M. G. H. The preparation of some bulky dihalo-, halohydrido- and dihydro- phosphineplatinum(ii) compounds. J. Organomet. Chem. 110, 271–279 (1976).
Acknowledgements
We thank the Royal Society and the European Research Council (FluoroFix: 677367) for funding, Johnson Matthey for the gift of PdCl2, and ANSTO for allocation of neutron beam time on KOALA to proposal P6932. We thank Oxford Cryosystems Ltd and ANSTO for funding a Russell Studentship with the University of Oxford (GAS).
Author information
Authors and Affiliations
Contributions
M.G. and C.B. carried out the synthetic studies. M.G. conducted DFT, QTAIM and related calculations. A.J.P.W. collected, processed and refined single-crystal X-ray diffraction data. A.J.E., R.I.C. and G.A.S. undertook the single-crystal Laue neutron diffraction experiment, data reduction and refined the neutron diffraction structural model. M.R.C. managed the project. All authors contributed to the writing and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Peer review information Nature thanks Jean-François Halet and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Fig. 1 Synthesis of group-10 hydride complexes with magnesium ligands.
a–e, Synthetic schemes for the preparation of complexes 1a (a), 2a (b), 2b (c), 2c (d) and 3 (e); for 3, the hydride ligands are derived from the C–H bonds of the benzene solvent.
Supplementary information
Supplementary Information
This file contains Supplementary Figures S1–S28 and Supplementary Tables S1–S12.
Supplementary Data
This cif file contains Neutron Diffraction Data.
Supplementary Data
This cif file contains X-ray Diffraction Data.
Rights and permissions
About this article
Cite this article
Garçon, M., Bakewell, C., Sackman, G.A. et al. A hexagonal planar transition-metal complex. Nature 574, 390–393 (2019). https://doi.org/10.1038/s41586-019-1616-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1616-2