Abstract
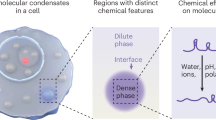
A passive consequence of macromolecular condensation is the establishment of an ion concentration gradient between the dilute and dense phases, which in turn governs distinct electrochemical properties of condensates. However, the mechanisms that regulate the electrochemical equilibrium of condensates and their impacts on emergent physicochemical functions remain unknown. Here we demonstrate that the electrochemical environments and the physical and chemical activities of biomolecular condensates, dependent on the electrochemical potential of condensates, are regulated by aging-associated intermolecular interactions and interfacial effects. Our findings reveal that enhanced dense-phase interactions during condensate maturation continuously modulate the ion distribution between the two phases. Moreover, modulating the interfacial regions of condensates can affect the apparent pH within the condensates. To directly probe the interphase and interfacial electric potentials of condensates, we have designed and implemented electrochemical potentiometry and second harmonic generation-based approaches. Our results suggest that the non-equilibrium nature of biomolecular condensates might play a crucial role in modulating the electrochemical activities of living systems.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All relevant data are included with the paper and its related Supplementary Information. Source data are provided with this paper.
References
Roden, C. & Gladfelter, A. S. RNA contributions to the form and function of biomolecular condensates. Nat. Rev. Mol. Cell Biol. 22, 183–195 (2021).
Banani, S. F., Lee, H. O., Hyman, A. A. & Rosen, M. K. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 (2017).
Alberti, S. & Hyman, A. A. Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat. Rev. Mol. Cell Biol. 22, 196–213 (2021).
Dai, Y., You, L. & Chilkoti, A. Engineering synthetic biomolecular condensates. Nat. Rev. Bioeng. 1, 466–480 (2023).
Choi, J.-M., Holehouse, A. S. & Pappu, R. V. Physical principles underlying the complex biology of intracellular phase transitions. Annu. Rev. Biophys. 49, 107–133 (2020).
Pappu, R. V., Cohen, S. R., Dar, F., Farag, M. & Kar, M. Phase transitions of associative biomacromolecules. Chem. Rev. 123, 8945–8987 (2023).
Romero-Perez, P. S., Dorone, Y., Flores, E., Sukenik, S. & Boeynaems, S. When phased without water: biophysics of cellular desiccation, from biomolecules to condensates. Chem. Rev. 123, 9010–9035 (2023).
Posey, A. E. et al. Biomolecular condensates are characterized by interphase electric potentials. J. Am. Chem. Soc. 10.1021/jacs.4c08946 (2024).
Dai, Y. et al. Biomolecular condensates regulate cellular electrochemical equilibria. Cell 187, 5951–5966.e5918 (2024).
Dai, Y. et al. Interface of biomolecular condensates modulates redox reactions. Chem 9, 1594–1609 (2023).
Kilgore, H. R. & Young, R. A. Learning the chemical grammar of biomolecular condensates. Nat. Chem. Biol. 18, 1298–1306 (2022).
Kilgore, H. R. et al. Distinct chemical environments in biomolecular condensates. Nat. Chem. Biol. 20, 291–301 (2024).
Ye, S. et al. Micropolarity governs the structural organization of biomolecular condensates. Nat. Chem. Biol. 20, 443–451 (2024).
Cakmak, F. P., Choi, S., Meyer, M. O., Bevilacqua, P. C. & Keating, C. D. Prebiotically-relevant low polyion multivalency can improve functionality of membraneless compartments. Nat. Commun. 11, 5949 (2020).
Choi, S., Knoerdel, A. R., Sing, C. E. & Keating, C. D. Effect of polypeptide complex coacervate microenvironment on protonation of a guest molecule. J. Phys. Chem. B 127, 5978–5991 (2023).
Ausserwöger, H. et al. Quantifying collective interactions in biomolecular phase separation. Preprint at https://doi.org/10.1101/2023.05.31.543137 (2023).
King, M. R. et al. Macromolecular condensation organizes nucleolar sub-phases to set up a pH gradient. Cell 187, 1889–1906.e1824 (2024).
Watson, J. L. et al. Macromolecular condensation buffers intracellular water potential. Nature 623, 842–852 (2023).
Iglesias-Artola, J. M. et al. Charge-density reduction promotes ribozyme activity in RNA–peptide coacervates via RNA fluidization and magnesium partitioning. Nat. Chem. 14, 407–416 (2022).
Choi, S., Meyer, M. O., Bevilacqua, P. C. & Keating, C. D. Phase-specific RNA accumulation and duplex thermodynamics in multiphase coacervate models for membraneless organelles. Nat. Chem. 14, 1110–1117 (2022).
Cao, S. et al. Dipeptide coacervates as artificial membraneless organelles for bioorthogonal catalysis. Nat. Commun. 15, 39 (2024).
Guo, X. et al. Biomolecular condensates can function as inherent catalysts. Preprint at https://doi.org/10.1101/2024.07.06.602359 (2024).
Hoffmann, C. et al. Electric potential at the interface of membraneless organelles gauged by graphene. Nano Lett. 23, 10796–10801 (2023).
Yan, X. et al. Intra-condensate demixing of TDP-43 inside stress granules generates pathological aggregates. Preprint at https://doi.org/10.1101/2024.01.23.576837 (2024).
Dai, Y., Wang, Z.-G. & Zare, R. N. Unlocking the electrochemical functions of biomolecular condensates. Nat. Chem. Biol. 20, 1420–1433 (2024).
Lee, D.-yD. et al. Magnesium flux modulates ribosomes to increase bacterial survival. Cell 177, 352–360.e313 (2019).
Prindle, A. et al. Ion channels enable electrical communication in bacterial communities. Nature 527, 59–63 (2015).
Kikuchi, K. et al. Electrochemical potential enables dormant spores to integrate environmental signals. Science 378, 43–49 (2022).
Shin, Y. & Brangwynne, C. P. Liquid phase condensation in cell physiology and disease. Science 357, eaaf4382 (2017).
Wolozin, B. & Ivanov, P. Stress granules and neurodegeneration. Nat. Rev. Neurosci. 20, 649–666 (2019).
Tsang, B., Pritišanac, I., Scherer, S. W., Moses, A. M. & Forman-Kay, J. D. Phase separation as a missing mechanism for interpretation of disease mutations. Cell 183, 1742–1756 (2020).
Brangwynne, C. P., Tompa, P. & Pappu, R. V. Polymer physics of intracellular phase transitions. Nat. Phys. 11, 899–904 (2015).
Erkamp, N. A. et al. Spatially non-uniform condensates emerge from dynamically arrested phase separation. Nat. Commun. 14, 684 (2023).
Galvanetto, N. et al. Extreme dynamics in a biomolecular condensate. Nature 619, 876–883 (2023).
Fritsch, A. W. et al. Local thermodynamics govern formation and dissolution of Caenorhabditis elegans P granule condensates. Proc. Natl Acad. Sci. USA 118, e2102772118 (2021).
Kirschbaum, J. & Zwicker, D. Controlling biomolecular condensates via chemical reactions. J. R. Soc. Interface 18, 20210255 (2021).
Takaki, R., Jawerth, L., Popović, M. & Jülicher, F. Theory of rheology and aging of protein condensates. PRX Life 1, 013006 (2023).
Alshareedah, I. et al. Sequence-specific interactions determine viscoelasticity and ageing dynamics of protein condensates. Nat. Phys 20, 1482–1491 (2024).
Alshareedah, I., Moosa, M. M., Pham, M., Potoyan, D. A. & Banerjee, P. R. Programmable viscoelasticity in protein–RNA condensates with disordered sticker-spacer polypeptides. Nat. Commun. 12, 6620 (2021).
Holehouse, A. S. & Kragelund, B. B. The molecular basis for cellular function of intrinsically disordered protein regions. Nat. Rev. Mol. Cell Biol. 25, 187–211 (2024).
Nott, T. J. et al. Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol. Cell 57, 936–947 (2015).
Dai, Y. et al. Programmable synthetic biomolecular condensates for cellular control. Nat. Chem. Biol. 19, 518–528 (2023).
Dzuricky, M., Rogers, B. A., Shahid, A., Cremer, P. S. & Chilkoti, A. De novo engineering of intracellular condensates using artificial disordered proteins. Nat. Chem. 12, 814–825 (2020).
Tan, C., Saurabh, S., Bruchez, M. P., Schwartz, R. & LeDuc, P. Molecular crowding shapes gene expression in synthetic cellular nanosystems. Nat. Nanotechnol. 8, 602–608 (2013).
Shen, Y. et al. The liquid-to-solid transition of FUS is promoted by the condensate surface. Proc. Natl Acad. Sci. USA 120, e2301366120 (2023).
Emmanouilidis, L. et al. NMR and EPR reveal a compaction of the RNA-binding protein FUS upon droplet formation. Nat. Chem. Biol. 17, 608–614 (2021).
Emmanouilidis, L. et al. A solid beta-sheet structure is formed at the surface of FUS droplets during aging. Nat. Chem. Biol. 20, 1044–1052 (2024).
Farag, M. et al. Condensates formed by prion-like low-complexity domains have small-world network structures and interfaces defined by expanded conformations. Nat. Commun. 13, 7722 (2022).
Linsenmeier, M. et al. The interface of condensates of the hnRNPA1 low-complexity domain promotes formation of amyloid fibrils. Nat. Chem. 15, 1340–1349 (2023).
Mangiarotti, A., Chen, N., Zhao, Z., Lipowsky, R. & Dimova, R. Wetting and complex remodeling of membranes by biomolecular condensates. Nat. Commun. 14, 2809 (2023).
Chen, B. et al. Water–solid contact electrification causes hydrogen peroxide production from hydroxyl radical recombination in sprayed microdroplets. Proc. Natl Acad. Sci. USA 119, e2209056119 (2022).
Lin, S., Cao, L. N. Y., Tang, Z. & Wang, Z. L. Size-dependent charge transfer between water microdroplets. Proc. Natl Acad. Sci. USA 120, e2307977120 (2023).
Trefalt, G., Behrens, S. H. & Borkovec, M. Charge regulation in the electrical double layer: ion adsorption and surface interactions. Langmuir 32, 380–400 (2016).
Vis, M. et al. Effects of electric charge on the interfacial tension between coexisting aqueous mixtures of polyelectrolyte and neutral polymer. Macromolecules 48, 7335–7345 (2015).
Zhang, P. & Wang, Z.-G. Interfacial structure and tension of polyelectrolyte complex coacervates. Macromolecules 54, 10994–11007 (2021).
Chen, G. Donnan equilibrium revisited: coupling between ion concentrations, osmotic pressure and Donnan potential. J. Micromech. Mol. Phys. 7, 127–134 (2022).
Vis, M., Peters, V. F., Tromp, R. H. & Erné, B. H. Donnan potentials in aqueous phase-separated polymer mixtures. Langmuir 30, 5755–5762 (2014).
Stepanov, V. P. & Kulik, N. P. Galvani potential at liquid-liquid interfaces for dissolving AgBr + LiCl and AgI + LiCl melts. Ionics 24, 2851–2856 (2018).
Li, L. et al. Phase behavior and salt partitioning in polyelectrolyte complex coacervates. Macromolecules 51, 2988–2995 (2018).
Iqbal, M. et al. Aqueous two-phase system (ATPS): an overview and advances in its applications. Biol. Proc. Online 18, 18 (2016).
Haynes, C. A., Carson, J., Blanch, H. W. & Prausnitz, J. M. Electrostatic potentials and protein partitioning in aqueous two-phase systems. AlChE J. 37, 1401–1409 (1991).
Bard, A. J., Faulkner, L. R. & White, H. S. Electrochemical Methods: Fundamentals and Applications (Wiley, 2022).
Lamoureux, G. & Roux, B. Absolute hydration free energy scale for alkali and halide ions established from simulations with a polarizable force field. J. Phys. Chem. B 110, 3308–3322 (2006).
Criss, C. M. & Luksha, E. Thermodynamic properties of nonaqueous solutions. IV. Free energies and entropies of solvation of some alkali metal halides in N,N-dimethylformamide. J. Phys. Chem. 72, 2966–2970 (1968).
Dzbek, J. & Korzeniewski, B. Control over the contribution of the mitochondrial membrane potential (ΔΨ) and proton gradient (ΔpH) to the protonmotive force (Δp): in silico studies. J. Biol. Chem. 283, 33232–33239 (2008).
Eisenthal, K. B. Second harmonic spectroscopy of aqueous nano- and microparticle interfaces. Chem. Rev. 106, 1462–1477 (2006).
Yan, E. C. Y., Liu, Y. & Eisenthal, K. B. New method for determination of surface potential of microscopic particles by second harmonic generation. J. Phys. Chem. B 102, 6331–6336 (1998).
Corn, R. M. & Higgins, D. A. Optical second harmonic generation as a probe of surface chemistry. Chem. Rev. 94, 107–125 (1994).
Didier, M. E. P., Tarun, O. B., Jourdain, P., Magistretti, P. & Roke, S. Membrane water for probing neuronal membrane potentials and ionic fluxes at the single cell level. Nat. Commun. 9, 5287 (2018).
Jin, L. et al. Characterization and application of a new optical probe for membrane lipid domains. Biophys. J. 90, 2563–2575 (2006).
Liu, Y., Yan, E. C. Y., Zhao, X. & Eisenthal, K. B. Surface potential of charged liposomes determined by second harmonic generation. Langmuir 17, 2063–2066 (2001).
Zhao, X., Ong, S. & Eisenthal, K. B. Polarization of water molecules at a charged interface. Second harmonic studies of charged monolayers at the air/water interface. Chem. Phys. Lett. 202, 513–520 (1993).
Ong, S., Zhao, X. & Eisenthal, K. B. Polarization of water molecules at a charged interface: second harmonic studies of the silica/water interface. Chem. Phys. Lett. 191, 327–335 (1992).
Israelachvili, J. N. Intermolecular and Surface Forces (Academic Press, 2011).
Xing, D. et al. Capture of hydroxyl radicals by hydronium cations in water microdroplets. Angew. Chem. Int. Ed. 61, e202207587 (2022).
Finkelstein, E., Rosen, G. M. & Rauckman, E. J. Spin trapping of superoxide and hydroxyl radical: practical aspects. Arch. Biochem. Biophys. 200, 1–16 (1980).
Majee, A., Weber, C. A. & Jülicher, F. Charge separation at liquid interfaces. Phys. Rev. Res. 6, 033138 (2024).
Lhee, S. et al. Spatial localization of charged molecules by salt ions in oil-confined water microdroplets. Sci. Adv. 6, eaba0181 (2020).
Campioni, S. et al. The presence of an air-water interface affects formation and elongation of α-synuclein fibrils. J. Am. Chem. Soc. 136, 2866–2875 (2014).
Humphries, J. et al. Species-independent attraction to biofilms through electrical signaling. Cell 168, 200–209.e212 (2017).
Jentsch, T. J., Hübner, C. A. & Fuhrmann, J. C. Ion channels: function unravelled by dysfunction. Nat. Cell Biol. 6, 1039–1047 (2004).
Söding, J., Zwicker, D., Sohrabi-Jahromi, S., Boehning, M. & Kirschbaum, J. Mechanisms for active regulation of biomolecular condensates. Trends Cell Biol. 30, 4–14 (2020).
Simon, J., van Spanning, R. J. M. & Richardson, D. J. The organisation of proton motive and non-proton motive redox loops in prokaryotic respiratory systems. Biochim. Biophys. Acta 1777, 1480–1490 (2008).
Yan, E. C., Fu, L., Wang, Z. & Liu, W. Biological macromolecules at interfaces probed by chiral vibrational sum frequency generation spectroscopy. Chem. Rev. 114, 8471–8498 (2014).
Lyklema, J. Interfacial potentials: measuring the immeasurable? Substantia 1, 75–93 (2017).
Gonella, G. et al. Water at charged interfaces. Nat. Rev. Chem. 5, 466–485 (2021).
Guggenheim, E. The thermodynamics of interfaces in systems of several components. Trans. Faraday Soc. 35, 397–412 (1940).
Zukoski, C. IV & Saville, D. The interpretation of electrokinetic measurements using a dynamic model of the stern layer: I. The dynamic model. J. Colloid Interface Sci. 114, 32–44 (1986).
Welsh, T. J. et al. Surface electrostatics govern the emulsion stability of biomolecular condensates. Nano Lett. 22, 612–621 (2022).
Kwok, K. Y., Mckenzie, D. L., Evers, D. L. & Rice, K. G. Formulation of highly soluble poly (ethylene glycol)‐peptide DNA condensates. J. Pharm. Sci. 88, 996–1003 (1999).
Lyklema, J. & Overbeek, J. T. G. On the interpretation of electrokinetic potentials. J. Colloid Sci. 16, 501–512 (1961).
Morelli, C. et al. RNA modulates hnRNPA1A amyloid formation mediated by biomolecular condensates. Nat. Chem. 16, 1052–1061 (2024).
Joshi, A. et al. Hydrogen-bonded network of water in phase-separated biomolecular condensates. J. Phys. Chem. Lett. 15, 7724–7734 (2024).
Quiroz, F. G. & Chilkoti, A. Sequence heuristics to encode phase behaviour in intrinsically disordered protein polymers. Nat. Mater. 14, 1164–1171 (2015).
Meyer, D. E. & Chilkoti, A. Purification of recombinant proteins by fusion with thermally-responsive polypeptides. Nat. Biotechnol. 17, 1112–1115 (1999).
King, R. S., Blanch, H. W. & Prausnitz, J. M. Molecular thermodynamics of aqueous two-phase systems for bioseparations. AlChE J. 34, 1585–1594 (1988).
Knowles, T. P. J. et al. Kinetics and thermodynamics of amyloid formation from direct measurements of fluctuations in fibril mass. Proc. Natl Acad. Sci. USA 104, 10016–10021 (2007).
Meisl, G. et al. Molecular mechanisms of protein aggregation from global fitting of kinetic models. Nat. Protoc. 11, 252–272 (2016).
Acknowledgements
We acknowledge experimental support from the Center for Biomolecular Condensates and the Biology Imaging Facility of Washington University in St Louis. We appreciate helpful discussions with Z.-G. Wang’s group at California Institute of Technology. We acknowledge funding support from the McKelvy School of Engineering of Washington University in St Louis. Part of this work was supported by the Air Force Office of Scientific Research (FA9550-21-1-0170 to R.N.Z.).
Author information
Authors and Affiliations
Contributions
Y.D. generated the idea for this article. Y.D., G.H. and R.N.Z. devised the study. W.Y., X.G., Y.X., Y.M., Z.T., L.Y. and X.S. conducted the experiments. X.G., Y.X., W.Y., Y.M., L.Y. and Z.T. analysed the data. Y.D., G.H. and R.N.Z. wrote the paper, and all authors commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Ellen Adams, Ben Erne, Drgomir Milovanovic, Mario Tagliazucchi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Texts 1–3, Figs. 1–7, Table 1 and References.
Source data
Source Data Fig. 1
Interior apparent pH of condensates at different time points.
Source Data Fig. 2
Different categories of condensates or treatments to condensates and their corresponding interior apparent pH.
Source Data Fig. 3
Electrochemical quantification of interphase electric potential difference between phases for different salt conditions and aging process.
Source Data Fig. 4
SHG signal of condensates under different salt conditions and aging time points and derived surface potentials of condensates at different ages.
Source Data Fig. 5
Analysis of electrochemical activity, partitioning functions of charged molecules and the growth of amyloid based on condensates with different ages.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, W., Guo, X., Xia, Y. et al. Aging-dependent evolving electrochemical potentials of biomolecular condensates regulate their physicochemical activities. Nat. Chem. (2025). https://doi.org/10.1038/s41557-025-01762-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41557-025-01762-7