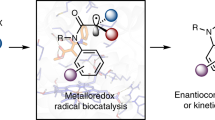
Abstract
Catalytic asymmetric dearomatization represents a powerful means to convert flat aromatic compounds into stereochemically well-defined three-dimensional molecular scaffolds. Using new-to-nature metalloredox biocatalysis, we describe an enzymatic strategy for catalytic asymmetric dearomatization via a challenging radical mechanism that has eluded small-molecule catalysts. Enabled by directed evolution, new-to-nature radical dearomatases P450rad1–P450rad5 facilitated asymmetric dearomatization of a broad spectrum of aromatic substrates, including indoles, pyrroles and phenols, allowing both enantioconvergent and enantiodivergent radical dearomatization reactions to be accomplished with excellent enzymatic control. Computational studies revealed the importance of additional hydrogen bonding interactions between the engineered metalloenzyme and the reactive intermediate in enhancing enzymatic activity and enantiocontrol. Furthermore, designer non-ionic surfactants were found to significantly accelerate this biotransformation, providing an alternative means to promote otherwise sluggish new-to-nature biotransformations. Together, this evolvable metalloenzyme platform opens up new avenues to advance challenging catalytic asymmetric dearomatization processes involving free radical intermediates.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data are available in the main text and the Supplementary Information. Crystallographic data for compounds 2e, 4a, 6a and 8a reported in this Article have been deposited at the CCDC under deposition numbers 2245164 (2e), 2245162 (4a), 2245161 (6a) and 2245163 (8a). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. The raw data for the docking structures used in the Supplementary Information are also available from the authors upon reasonable request. Plasmids encoding evolved radical dearomatases reported in this study are available for research purposes from Y.Y. under a material transfer agreement with the University of California, Santa Barbara.
References
von Ragué Schleyer, P. Introduction: aromaticity. Chem. Rev. 101, 1115–1118 (2001).
Zheng, C. & You, S.-L. Catalytic asymmetric dearomatization (CADA) reaction-enabled total synthesis of indole-based natural products. Nat. Prod. Rep. 36, 1589–1605 (2019).
Wertjes, W. C., Southgate, E. H. & Sarlah, D. Recent advances in chemical dearomatization of nonactivated arenes. Chem. Soc. Rev. 47, 7996–8017 (2018).
Zhuo, C.-X., Zhang, W. & You, S.-L. Catalytic asymmetric dearomatization reactions. Angew. Chem. Int. Ed. 51, 12662–12686 (2012).
Zheng, C. & You, S.-L. Advances in catalytic asymmetric dearomatization. ACS Cent. Sci. 7, 432–444 (2021).
Roche, S. P. & Porco, J. A. Jr. Dearomatization strategies in the synthesis of complex natural products. Angew. Chem. Int. Ed. 50, 4068–4093 (2011).
Wang, Y., Zhang, W.-Y., Yu, Z.-L., Zheng, C. & You, S.-L. SmI2-mediated enantioselective reductive dearomatization of non-activated arenes. Nat. Synth. 1, 401–406 (2022).
Zhang, W.-Y., Wang, H.-C., Wang, Y., Zheng, C. & You, S.-L. Enantioselective dearomatization of indoles via SmI2-mediated intermolecular reductive coupling with ketones. J. Am. Chem. Soc. 145, 10314–10321 (2023).
Proctor, R. S. J., Colgan, A. C. & Phipps, R. J. Exploiting attractive non-covalent interactions for the enantioselective catalysis of reactions involving radical intermediates. Nat. Chem. 12, 990–1004 (2020).
Mondal, S. et al. Enantioselective radical reactions using chiral catalysts. Chem. Rev. 122, 5842–5976 (2022).
Sibi, M. P., Manyem, S. & Zimmerman, J. Enantioselective radical processes. Chem. Rev. 103, 3263–3296 (2003).
Bloom, J. D. & Arnold, F. H. In the light of directed evolution: pathways of adaptive protein evolution. Proc. Natl Acad. Sci. USA 106, 9995–10000 (2009).
Reetz, M. T. Laboratory evolution of stereoselective enzymes: a prolific source of catalysts for asymmetric reactions. Angew. Chem. Int. Ed. 50, 138–174 (2011).
Bornscheuer, U. T. et al. Engineering the third wave of biocatalysis. Nature 485, 185–194 (2012).
Gibson, D. T., Koch, J. R. & Kallio, R. E. Oxidative degradation of aromatic hydrocarbons by microorganisms. I. Enzymic formation of catechol from benzene. Biochemistry 7, 2653–2662 (1968).
Boyd, D. R. & Bugg, T. D. H. Arene cis-dihydrodiol formation: from biology to application. Org. Biomol. Chem. 4, 181–192 (2006).
Johnson, R. A. Microbial arene oxidations. In Organic Reactions (ed. Overman, L. E.) 117–264 (John Wiley & Sons, 2004).
Baker Dockrey, S. A., Lukowski, A. L., Becker, M. R. & Narayan, A. R. H. Biocatalytic site- and enantioselective oxidative dearomatization of phenols. Nat. Chem. 10, 119–125 (2018).
Jacoby, C. et al. Channeling C1 metabolism toward S-adenosylmethionine-dependent conversion of estrogens to androgens in estrogen-degrading bacteria. mBio 11, https://doi.org/10.1128/mbio.01259-20 (2020).
Zhou, Q., Chin, M., Fu, Y., Liu, P. & Yang, Y. Stereodivergent atom-transfer radical cyclization by engineered cytochromes P450. Science 374, 1612–1616 (2021).
Fu, Y. et al. Engineered P450 atom-transfer radical cyclases are bifunctional biocatalysts: reaction mechanism and origin of enantioselectivity. J. Am. Chem. Soc. 144, 13344–13355 (2022).
Rui, J. et al. Directed evolution of nonheme iron enzymes to access abiological radical-relay C(sp3)−H azidation. Science 376, 869–874 (2022).
Fu, W. et al. Enzyme-controlled stereoselective radical cyclization to arenes enabled by metalloredox biocatalysis. Nat. Catal. 6, 628–636 (2023).
Emmanuel, M. A. et al. Photobiocatalytic strategies for organic synthesis. Chem. Rev. 123, 5459–5520 (2023).
Emmanuel, M. A., Greenberg, N. R., Oblinsky, D. G. & Hyster, T. K. Accessing non-natural reactivity by irradiating nicotinamide-dependent enzymes with light. Nature 540, 414–417 (2016).
Biegasiewicz, K. F. et al. Photoexcitation of flavoenzymes enables a stereoselective radical cyclization. Science 364, 1166–1169 (2019).
Black, M. J. et al. Asymmetric redox-neutral radical cyclization catalysed by flavin-dependent ‘ene’-reductases. Nat. Chem. 12, 71–75 (2020).
Clayman, P. D. & Hyster, T. K. Photoenzymatic generation of unstabilized alkyl radicals: an asymmetric reductive cyclization. J. Am. Chem. Soc. 142, 15673–15677 (2020).
Page, C. G. et al. Quaternary charge-transfer complex enables photoenzymatic intermolecular hydroalkylation of olefins. J. Am. Chem. Soc. 143, 97–102 (2021).
Gao, X., Turek-Herman, J. R., Choi, Y. J., Cohen, R. D. & Hyster, T. K. Photoenzymatic synthesis of α-tertiary amines by engineered flavin-dependent “ene”-reductases. J. Am. Chem. Soc. 143, 19643–19647 (2021).
Nicholls, B. T. et al. Engineering a non-natural photoenzyme for improved photon efficiency. Angew. Chem. Int. Ed. 61, e202113842 (2022).
Fu, H. et al. An asymmetric sp3–sp3 cross-electrophile coupling using ‘ene’-reductases. Nature 610, 302–307 (2022).
Fu, H., Qiao, T., Carceller, J. M., MacMillan, S. N. & Hyster, T. K. Asymmetric C-alkylation of nitroalkanes via enzymatic photoredox catalysis. J. Am. Chem. Soc. 145, 787–793 (2023).
Page, C. G. et al. Regioselective radical alkylation of arenes using evolved photoenzymes. J. Am. Chem. Soc. 145, 11866–11874 (2023).
Harrison, W., Huang, X. & Zhao, H. Photobiocatalysis for abiological transformations. Acc. Chem. Res. 55, 1087–1096 (2022).
Huang, X. et al. Photoenzymatic enantioselective intermolecular radical hydroalkylation. Nature 584, 69–74 (2020).
Huang, X. et al. Photoinduced chemomimetic biocatalysis for enantioselective intermolecular radical conjugate addition. Nat. Catal. 5, 586–593 (2022).
Cheng, L. et al. Stereoselective amino acid synthesis by synergistic photoredox-pyridoxal radical biocatalysis. Science 381, 444–451 (2023).
Quasdorf, K. W. & Overman, L. E. Catalytic enantioselective synthesis of quaternary carbon stereocentres. Nature 516, 181–191 (2014).
Süsse, L. & Stoltz, B. M. Enantioselective formation of quaternary centers by allylic alkylation with first-row transition-metal catalysts. Chem. Rev. 121, 4084–4099 (2021).
Poulos, T. L. Heme enzyme structure and function. Chem. Rev. 114, 3919–3962 (2014).
Phillips, I. R., Shephard, E. A. & Ortiz de Montellano, P. R. Cytochrome P450 Protocols (Humana, 2013).
Brandenberg, O. F., Fasan, R. & Arnold, F. H. Exploiting and engineering hemoproteins for abiological carbene and nitrene transfer reactions. Curr. Opin. Biotechnol. 47, 102–111 (2017).
Yang, Y. & Arnold, F. H. Navigating the unnatural reaction space: directed evolution of heme proteins for selective carbene and nitrene transfer. Acc. Chem. Res. 54, 1209–1225 (2021).
Mukaiyama, T., Ogata, K., Sato, I. & Hayashi, Y. Asymmetric organocatalyzed Michael addition of nitromethane to a 2-oxoindoline-3-ylidene acetaldehyde and the three one-pot sequential synthesis of (−)-horsfiline and (−)-coerulescine. Chem. Eur. J. 20, 13583–13588 (2014).
Díaz-Marrero, A. R. et al. Carijodienone from the octocoral Carijoa multiflora. A spiropregnane-based steroid. J. Nat. Prod. 74, 292–295 (2011).
Bandini, M. & Eichholzer, A. Catalytic functionalization of indoles in a new dimension. Angew. Chem. Int. Ed. 48, 9608–9644 (2009).
Brandenberg, O. F. et al. Stereoselective enzymatic synthesis of heteroatom-substituted cyclopropanes. ACS Catal. 8, 2629–2634 (2018).
McIntosh, J. A. et al. Enantioselective intramolecular C—H amination catalyzed by engineered cytochrome P450 enzymes in vitro and in vivo. Angew. Chem. Int. Ed. 52, 9309–9312 (2013).
Højgaard, C., Sørensen, H. V., Pedersen, J. S., Winther, J. R. & Otzen, D. E. Can a charged surfactant unfold an uncharged protein? Biophys. J. 115, 2081–2086 (2018).
Cortes-Clerget, M. et al. MC-1. A “designer” surfactant engineered for peptide synthesis in water at room temperature. Green Chem. 21, 2610–2614 (2019).
Lipshutz, B. H. et al. TPGS-750-M: a second-generation amphiphile for metal-catalyzed cross-couplings in water at room temperature. J. Org. Chem. 76, 4379–4391 (2011).
Klumphu, P. & Lipshutz, B. H. “Nok”: a phytosterol-based amphiphile enabling transition-metal-catalyzed couplings in water at room temperature. J. Org. Chem. 79, 888–900 (2014).
Noey, E. L. et al. Origins of stereoselectivity in evolved ketoreductases. Proc. Natl Acad. Sci. USA 112, E7065–E7072 (2015).
Xu, R.-Q., Yang, P., Tu, H.-F., Wang, S.-G. & You, S.-L. Palladium(0)-catalyzed intermolecular arylative dearomatization of β-naphthols. Angew. Chem. Int. Ed. 55, 15137–15141 (2016).
Hu, J. et al. Pd-catalyzed dearomative asymmetric allylic alkylation of naphthols with alkoxyallenes. J. Org. Chem. 85, 7896–7904 (2020).
Mohamed, M. A., Yamada, K. & Tomioka, K. Accessing the amide functionality by the mild and low-cost oxidation of imine. Tetrahedron Lett. 50, 3436–3438 (2009).
Wang, G., Lu, R., He, C. & Liu, L. Kinetic resolution of indolines by asymmetric hydroxylamine formation. Nat. Commun. 12, 2512 (2021).
Acknowledgements
This research is supported by the National Institutes of Health (NIH; R35GM147387 to Y.Y. and R35GM128779 to P.L.). We acknowledge the National Science Foundation (NSF) BioPolymers, Automated Cellular Infrastructure, Flow, and Integrated Chemistry Materials Innovation Platform (BioPACIFIC MIP; DMR-1933487) and the NSF Materials Research Science and Engineering Center (MRSEC) at the University of California, Santa Barbara (DMR-2308708) for access to instrumentation. MD simulations were performed at the Center for Research Computing of the University of Pittsburgh and the Advanced Cyberinfrastructure Coordination Ecosystem: Services & Support (ACCESS) programme supported by the NSF, grant numbers OAC-2117681 and OAC-2138259. Y.F. is an Andrew W. Mellon Predoctoral Fellow. We thank Y.-M. Wang (University of Pittsburgh) for critical reading of this manuscript and B. Lipshutz (University of California, Santa Barbara) for the generous donation of surfactants used in this study.
Author information
Authors and Affiliations
Contributions
Y.Y. conceived and directed the project. W.F., Y.Z. and H.W. prepared the substrates. W.F. and Y.Z. performed enzyme screening, enzyme engineering and substrate scope studies. Y.F. carried out the computational studies with P.L. providing guidance. Y.Y., P.L., W.F. and Y.F. wrote the manuscript with the input of all other authors.
Corresponding authors
Ethics declarations
Competing interests
Y.Y., W.F. and Y.Z. are inventors on a patent application (International Patent Application Serial No. PCT/US2023/085941) submitted by the University of California, Santa Barbara that covers compositions, methods and applications of evolved radical dearomatases. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Zhongyue Yang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–18, Tables 1–25, Discussion, Methods, analytical data, spectra and references.
Supplementary Data 1
Crystallographic data for compound 2e; CCDC reference 2245164.
Supplementary Data 2
Crystallographic data for compound 4a; CCDC reference 2245162.
Supplementary Data 3
Crystallographic data for compound 6a; CCDC reference 2245161.
Supplementary Data 4
Crystallographic data for compound 8a; CCDC reference 2245163.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fu, W., Fu, Y., Zhao, Y. et al. A metalloenzyme platform for catalytic asymmetric radical dearomatization. Nat. Chem. 16, 1999–2008 (2024). https://doi.org/10.1038/s41557-024-01608-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-024-01608-8