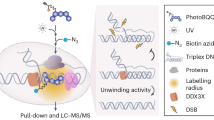
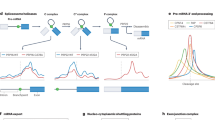
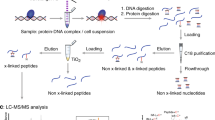
Abstract
Precise dissection of DNA–protein interactions is essential for elucidating the recognition basis, dynamics and gene regulation mechanism. However, global profiling of weak and dynamic DNA–protein interactions remains a long-standing challenge. Here, we establish the light-induced lysine (K) enabled crosslinking (LIKE-XL) strategy for spatiotemporal and global profiling of DNA–protein interactions. Harnessing unique abilities to capture weak and transient DNA–protein interactions, we demonstrate that LIKE-XL enables the discovery of low-affinity transcription-factor/DNA interactions via sequence-specific DNA baits, determining the binding sites for transcription factors that have been previously unknown. More importantly, we successfully decipher the dynamics of the transcription factor subproteome in response to drug treatment in a time-resolved manner, and find downstream target transcription factors from drug perturbations, providing insight into their dynamic transcriptional networks. The LIKE-XL strategy offers a complementary method to expand the DNA–protein profiling toolbox and map accurate DNA–protein interactomes that were previously inaccessible via non-covalent strategies, for better understanding of protein function in health and disease.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data in support of the findings of this study are available within the article and in the Supplementary Information. All relevant MS data have been deposited to the ProteomeXchange Consortium with the dataset identifier PXD039229. Source data are provided with this paper.
References
Hudson, W. H. & Ortlund, E. A. The structure, function and evolution of proteins that bind DNA and RNA. Nat. Rev. Mol. Cell Biol. 15, 749–760 (2014).
Venkatesh, S. & Workman, J. L. Histone exchange, chromatin structure and the regulation of transcription. Nat. Rev. Mol. Cell Biol. 16, 178–189 (2015).
Weirauch, M. T. et al. Determination and inference of eukaryotic transcription factor sequence specificity. Cell 158, 1431–1443 (2014).
Kribelbauer, J. F., Rastogi, C., Bussemaker, H. J. & Mann, R. S. Low-affinity binding sites and the transcription factor specificity paradox in eukaryotes. Annu. Rev. Cell Dev. Biol. 35, 357–379 (2019).
Jolma, A. et al. DNA-binding specificities of human transcription factors. Cell 152, 327–339 (2013).
Vidal, M., Cusick, Michael, E. & Barabási, A.-L. Interactome networks and human disease. Cell 144, 986–998 (2011).
Wierer, M. & Mann, M. Proteomics to study DNA-bound and chromatin-associated gene regulatory complexes. Hum. Mol. Genet. 25, R106–R114 (2016).
Stormo, G. D. & Zhao, Y. Determining the specificity of protein–DNA interactions. Nat. Rev. Genet. 11, 751–760 (2010).
Furey, T. S. ChIP–seq and beyond: new and improved methodologies to detect and characterize protein–DNA interactions. Nat. Rev. Genet. 13, 840–852 (2012).
Xie, Z., Hu, S., Qian, J., Blackshaw, S. & Zhu, H. Systematic characterization of protein-DNA interactions. Cell. Mol. Life Sci. 68, 1657–1668 (2011).
Déjardin, J. & Kingston, R. E. Purification of proteins associated with specific genomic loci. Cell 136, 175–186 (2009).
Williams, P., Li, L., Dong, X. & Wang, Y. Identification of SLIRP as a G quadruplex-binding protein. J. Am. Chem. Soc. 139, 12426–12429 (2017).
Li, L. et al. YY1 interacts with guanine quadruplexes to regulate DNA looping and gene expression. Nat. Chem. Biol. 17, 161–168 (2021).
Bartke, T. et al. Nucleosome-interacting proteins regulated by DNA and histone methylation. Cell 143, 470–484 (2010).
Spruijt, C. G. et al. Dynamic readers for 5-(hydroxy)methylcytosine and its oxidized derivatives. Cell 152, 1146–1159 (2013).
Zhou, Q. et al. A mouse tissue transcription factor atlas. Nat. Commun. 8, 15089 (2017).
Bai, L. et al. Proteome-wide profiling of readers for DNA modification. Adv. Sci. 8, 2101426 (2021).
Butter, F. et al. Proteome-wide analysis of disease-associated SNPs that show allele-specific transcription factor binding. PLoS Genet. 8, e1002982 (2012).
Mondal, S. et al. PROBER identifies proteins associated with programmable sequence-specific DNA in living cells. Nat. Methods 19, 959–968 (2022).
Cozzolino, F., Iacobucci, I., Monaco, V. & Monti, M. Protein–DNA/RNA interactions: an overview of investigation methods in the -omics era. J. Proteome Res. 20, 3018–3030 (2021).
Kantidze, O. L. & Razin, S. V. Weak interactions in higher-order chromatin organization. Nucleic Acids Res. 48, 4614–4626 (2020).
Duzdevich, D., Redding, S. & Greene, E. C. DNA dynamics and single-molecule biology. Chem. Rev. 114, 3072–3086 (2014).
Fuxreiter, M., Simon, I. & Bondos, S. Dynamic protein–DNA recognition: beyond what can be seen. Trends Biochem. Sci. 36, 415–423 (2011).
Steen, H. & Jensen, O. N. Analysis of protein–nucleic acid interactions by photochemical cross-linking and mass spectrometry. Mass Spectrom. Rev. 21, 163–182 (2002).
Hoffman, E. A., Frey, B. L., Smith, L. M. & Auble, D. T. Formaldehyde crosslinking: a tool for the study of chromatin complexes. J. Biol. Chem. 290, 26404–26411 (2015).
Gauchier, M., van Mierlo, G., Vermeulen, M. & Déjardin, J. Purification and enrichment of specific chromatin loci. Nat. Methods 17, 380–389 (2020).
Song, C.-X. & He, C. Bioorthogonal labeling of 5-hydroxymethylcytosine in genomic DNA and diazirine-based DNA photo-cross-linking probes. Acc. Chem. Res. 44, 709–717 (2011).
Trads, J. B., Tørring, T. & Gothelf, K. V. Site-selective conjugation of native proteins with DNA. Acc. Chem. Res. 50, 1367–1374 (2017).
van Mierlo, G. & Vermeulen, M. Chromatin proteomics to study epigenetics — challenges and opportunities. Mol. Cell. Proteom. 20, 100056 (2021).
Lercher, L., McGouran, J. F., Kessler, B. M., Schofield, C. J. & Davis, B. G. DNA modification under mild conditions by Suzuki–Miyaura cross-coupling for the generation of functional probes. Angew. Chem. Int. Ed. 52, 10553–10558 (2013).
Liu, Y. et al. Photoaffinity labeling of transcription factors by DNA-templated crosslinking. Chem. Sci. 6, 745–751 (2015).
Huang, Y. et al. Selection of DNA-encoded chemical libraries against endogenous membrane proteins on live cells. Nat. Chem. 13, 77–88 (2021).
Reim, A. et al. Atomic-resolution mapping of transcription factor-DNA interactions by femtosecond laser crosslinking and mass spectrometry. Nat. Commun. 11, 3019 (2020).
Stützer, A. et al. Analysis of protein-DNA interactions in chromatin by UV induced cross-linking and mass spectrometry. Nat. Commun. 11, 5250 (2020).
MacKinnon, A. L. & Taunton, J. Target identification by diazirine photo-cross-linking and click chemistry. Curr. Protoc. Chem. Biol. 1, 55–73 (2009).
Preston, G. W. & Wilson, A. J. Photo-induced covalent cross-linking for the analysis of biomolecular interactions. Chem. Soc. Rev. 42, 3289–3301 (2013).
Singh, Y., Murat, P. & Defrancq, E. Recent developments in oligonucleotide conjugation. Chem. Soc. Rev. 39, 2054–2070 (2010).
Ivancová, I., Leone, D.-L. & Hocek, M. Reactive modifications of DNA nucleobases for labelling, bioconjugations, and cross-linking. Curr. Opin. Chem. Biol. 52, 136–144 (2019).
Rosen, C. B. et al. Template-directed covalent conjugation of DNA to native antibodies, transferrin and other metal-binding proteins. Nat. Chem. 6, 804–809 (2014).
Ivancová, I., Pohl, R., Hubálek, M. & Hocek, M. Squaramate-modified nucleotides and DNA for specific cross-linking with lysine-containing peptides and proteins. Angew. Chem. Int. Ed. 58, 13345–13348 (2019).
Dadová, J. et al. Vinylsulfonamide and acrylamide modification of DNA for cross-linking with proteins. Angew. Chem. Int. Ed. 52, 10515–10518 (2013).
Dadová, J. et al. Azidopropylvinylsulfonamide as a new bifunctional click reagent for bioorthogonal conjugations: application for DNA–protein cross-linking. Chem. Eur. J. 21, 16091–16102 (2015).
Olszewska, A., Pohl, R., Brázdová, M., Fojta, M. & Hocek, M. Chloroacetamide-linked nucleotides and DNA for cross-linking with peptides and proteins. Bioconjugate Chem. 27, 2089–2094 (2016).
Krömer, M., Brunderová, M., Ivancová, I., Poštová Slavětínská, L. & Hocek, M. 2-Formyl-dATP as substrate for polymerase synthesis of reactive DNA bearing an aldehyde group in the minor groove. ChemPlusChem 85, 1164–1170 (2020).
Leone, D.-L., Hubálek, M., Pohl, R., Sýkorová, V. & Hocek, M. 1,3-Diketone-modified nucleotides and DNA for cross-linking with arginine-containing peptides and proteins. Angew. Chem. Int. Ed. 60, 17383–17387 (2021).
Yu, B., Pettitt, B. M. & Iwahara, J. Dynamics of ionic interactions at protein–nucleic acid interfaces. Acc. Chem. Res. 53, 1802–1810 (2020).
Rohs, R. et al. Origins of specificity in protein-DNA recognition. Annu. Rev. Biochem. 79, 233–269 (2010).
Murre, C. Helix–loop–helix proteins and the advent of cellular diversity: 30 years of discovery. Genes Dev. 33, 6–25 (2019).
Bürglin, T. R. & Affolter, M. Homeodomain proteins: an update. Chromosoma 125, 497–521 (2016).
Cuesta, A. & Taunton, J. Lysine-targeted inhibitors and chemoproteomic probes. Annu. Rev. Biochem. 88, 365–381 (2019).
Guo, A.-D. et al. Light-induced primary amines and o-nitrobenzyl alcohols cyclization as a versatile photoclick reaction for modular conjugation. Nat. Commun. 11, 5472 (2020).
Hu, W. et al. Genetically encoded residue-selective photo-crosslinker to capture protein-protein interactions in living cells. Chem 5, 2955–2968 (2019).
Yeldell, S. B. & Seitz, O. Nucleic acid constructs for the interrogation of multivalent protein interactions. Chem. Soc. Rev. 49, 6848–6865 (2020).
Gavins, G. C. et al. Live cell PNA labelling enables erasable fluorescence imaging of membrane proteins. Nat. Chem. 13, 15–23 (2021).
Maerkl, S. J. & Quake, S. R. A systems approach to measuring the binding energy landscapes of transcription factors. Science 315, 233–237 (2007).
Wei, G.-H. et al. Genome-wide analysis of ETS-family DNA-binding in vitro and in vivo. EMBO J. 29, 2147–2160 (2010).
Peart, M. J. et al. Identification and functional significance of genes regulated by structurally different histone deacetylase inhibitors. Proc. Natl Acad. Sci. USA 102, 3697–3702 (2005).
Li, W. & Sun, Z. Mechanism of action for HDAC inhibitors—insights from omics approaches. Int. J. Mol. Sci. 20, 1616 (2019).
Li, H. et al. The EMT regulator ZEB2 is a novel dependency of human and murine acute myeloid leukemia. Blood 129, 497–508 (2017).
Nagel, S. et al. Comprehensive analysis of homeobox genes in Hodgkin lymphoma cell lines identifies dysregulated expression of HOXB9 mediated via ERK5 signaling and BMI1. Blood 109, 3015–3023 (2007).
Wei, D. et al. Discovery of potent and selective CDK9 degraders for targeting transcription regulation in triple-negative breast cancer. J. Med. Chem. 64, 14822–14847 (2021).
Cidado, J. et al. AZD4573 is a highly selective CDK9 inhibitor that suppresses MCL-1 and induces apoptosis in hematologic cancer cells. Clin. Cancer Res. 26, 922–934 (2020).
West, A. V. et al. Labeling preferences of diazirines with protein biomolecules. J. Am. Chem. Soc. 143, 6691–6700 (2021).
Cravatt, B. F., Wright, A. T. & Kozarich, J. W. Activity-based protein profiling: from enzyme chemistry to proteomic chemistry. Annu. Rev. Biochem. 77, 383–414 (2008).
Fantoni, N. Z., El-Sagheer, A. H. & Brown, T. A hitchhiker’s guide to click-chemistry with nucleic acids. Chem. Rev. 121, 7122–7154 (2021)
Acknowledgements
We thank Y. Liu from Fudan University for assistance with the CUT&Tag sequencing data analysis. This study was supported by the National Science Foundation of China (92053106 to X.-H.C., 22225702 to M.T., 92153302 to M.T., 22177120 to X.-H.C., 81821005 to M.T. and 21907100 to H.H.); the National Key Science & Technology Program of China (2020YFE0202200 to M.T.); the Program of Shanghai Academic Research Leader (22XD1420900 to M.T.); the Innovative Research Team of High-Level Local Universities in Shanghai (SHSMU-ZDCX20212700 to M.T.); Youth Innovation Promotion Association of the Chinese Academy of Sciences (H.H.); and the Guangdong High-Level Innovative Research Institute (2021B0909050003 to M.T.).
Author information
Authors and Affiliations
Contributions
A.-D.G. performed the photo-crosslinking of DNA probes with TAMRA-NH2, peptides and recombinant TFs; crosslinking pull-down of probe-interacting proteins from nuclear extracts; western blots; and preparation of the RNA-seq sample and cell culture. K.-N.Y. modified the DNA probes, synthesized TAMRA-NH2, performed the photo-crosslinking of model reactions and DNA with histones and performed and analysed conjugation products. H.H. performed the proteomics sample preparation, LC-MS/MS experiments, MS data analysis and bioinformatics analysis. L.Z. assisted in the LC-MS/MS experiments and data analysis. T.-F.H. assisted in the photo-crosslinking of model reactions and analysed the conjugation products. H.S. and Y.X. performed the ITC assays. Y.C. and D.Z. performed the analysis of the CUT&Tag data. X.L. assisted in the MS analysis of the DNA probes. J. Zha and J. Zhang performed molecular docking. Y.-J.X. assisted in the data analysis. X.-H.C. and M.T. directed and supervised the project and analysed the data. X.-H.C., A.-D.G., K.-N.Y., H.H. and M.T. wrote the manuscript with collaboration from the other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Michal Hocek, Yinsheng Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Design of LIKE-XL strategy for temporal and global profiling of DNA-protein interactions.
a, Features of lysine side chain at protein-nucleic acid interfaces. b, Schematic of the LIKE-XL workflow for temporal and global profiling of DNA-protein interactions.
Extended Data Fig. 2 Comparison of o-NBA probe based covalent LIKE-XL pulldown and non-covalent affinity pulldown.
a, The schematics of pulldown workflow. Nuclear extracts were incubated with o-NBA probe (crosslinking pulldown and control 1) or control probe (control 2) for 30 min at 4 °C. Then, crosslinking pulldown and control 2 were treated with UV-365 irradiation for 15 min at 4 °C, and control 1 did not undergo light-treatment. All samples were incubated at 25 °C. for another 30 min, and subsequently for SDS-PAGE analysis or on-bead digestion followed by LC-MS/MS analysis. b, Silver staining. c, Overlap of identified proteins. d, MS signal intensity plots of identified proteins for each samples in o-NBA probe related experiments. e, The structure and sequence of ETS-TF o-NBA probe. f, Silver staining. g, Overlap of identified proteins. h, MS signal intensity plots of identified proteins for each samples in ETS-TF o-NBA probe related experiments. In d and h, Y-axis: log-transformed MS intensities, X-axis: protein number, and each dot represents a protein. The proteins were numbered according to intensity ranks, for example the one with the highest MS intensity is number 1#. Compared with crosslinking pulldown groups (P), there were much less bands (in silver staining, b, f) and proteins (from LC-MS/MS analysis, c, d, g, h) in two controls (C1 and C2). In addition, LIKE-XL pulldown performed at even much lower probe concentration (for example 5 μM) not only successfully retrieved the majority of proteins identified in non-covalent affinity pulldown (performed at 50 μM of probe), but also identified much more additional proteins.
Extended Data Fig. 3 Screening for target proteins of o-NBA probe.
Single-shot LC-MS/MS analysis identified 1,200–1,600 in each replicate. Venn diagrams show comparison of proteins identified from pulldown groups and competition groups for replicate 1 (a) and replicate 2 (c). The log2(pulldown/competition) of proteins commonly identified in both groups are shown in scatter plot (b and d). Proteins uniquely identified in pulldown groups (brown) or with log2(pulldown/competition) > 3 (red) are considered as ‘enriched proteins’ of o-NBA probe (e). That is, there are 229 and 223 proteins enriched by o-NBA probe in replicate 1 and 2, respectively. Overlap of the enriched proteins from replicate 1 and 2 are then considered as potential targets (n = 129) of o-NBA probe (see main text Fig. 4d).
Extended Data Fig. 4 Global profiling of specific DNA-TF interactions from HCT116 cells by o-NBA probe-based competitive crosslinking pulldown.
The experiments were performed in the same condition as in Fig. 4 in the main text. a, Silver staining of the pulldown and competition samples, with the red arrows indicating protein captured. b, Overlap of proteins enriched (for criterion, see Extended Data Fig. 3) in each duplicate. Proteins in the yellow-highlighted area (n = 112) are considered as target binders of the o-NBA probe. c, Fraction of target TFs of different types. d, PWM-energy based scores (a proxy of binding affinity) of target bHLH-TFs and HD- TFs.
Supplementary information
Supplementary Information
Supplementary Figs. 1–25, Tables 1 and 2, experimental details, synthesis and characterization data, and unprocessed scans of blots and gels for supplementary figures.
Supplementary Data
Supplementary Data 1. The o-NBA-probe-enriched proteins from the competitive crosslinking pull-down and TFs identified by RNA-seq (HeLa cells). Supplementary Data 2. The o-NBA-probe-enriched proteins from the competitive crosslinking pull-down (HCT116 cells). Supplementary Data 3. The Diz-probe-enriched proteins from the competitive crosslinking pull-down. Supplementary Data 4. The Azi-probe-enriched proteins from the competitive crosslinking pull-down. Supplementary Data 5. Comparison of specifically binding TFs by the o-NBA probe, Diz probe and Azi probe. Supplementary Data 6. Enriched TFs from SAHA-treated K562 cells. Supplementary Data 7. Enriched TFs from CDK9-degrader-treated MV-4-11 cells.
Source data
Source Data Fig. 2
Unprocessed gels.
Source Data Fig. 3
Unprocessed gels and statistical source data.
Source Data Fig. 4
Unprocessed gels.
Source Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 2
Unprocessed gels.
Source Data Extended Data Fig. 4
Unprocessed gels.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, AD., Yan, KN., Hu, H. et al. Spatiotemporal and global profiling of DNA–protein interactions enables discovery of low-affinity transcription factors. Nat. Chem. 15, 803–814 (2023). https://doi.org/10.1038/s41557-023-01196-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01196-z