Abstract
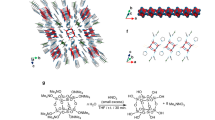
Polycyclic aromatic hydrocarbons (PAHs) show promise for applications in functional devices such as organic photovoltaics and field-effect transistors, but, although nanometre-sized PAHs—often referred to as nanographenes—have been well investigated as single-layer molecules, their multilayer counterparts remain rather unexplored. Here we show the assembly of a C64 nanographene derivative (comprising a planar core decorated with four meta-terphenyl–imide moieties at its periphery) into multilayer stacks with smaller PAHs ranging from naphthalene to ovalene and hexabenzocoronene. The functionalized C64 nanographene serves as a ditopic host that can accommodate a smaller PAH on either side of its planar core, in cavities delimited by its bulky imide substituents. Bilayers and trilayers (that is, complexes with 1:1 and 1:2 host:guest ratios, respectively) were observed in solution, and dimers of these complexes as well as multilayer compounds were isolated in the solid state. Quantum-chemical calculations indicate that dispersion forces are the main stabilizing factor for these complexes.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Crystallographic data for the structures in this Article have been deposited at the Cambridge Crystallographic Data Centre under deposition nos. CCDC 2068629 (monolayer, 1), 2068630 (multilayer, [COR·1·COR]n), 2068631 (hexalayer, [COR·1·COR]2) and 2068632 (tetralayer, [COR·1·1·COR]). Copies of data can be obtained free of charge from www.ccdc.cam.ac.uk/structures/. Details of the synthesis and crystallographic analyses, UV–vis and fluorescence spectra, traces of cyclic and differential pulse voltammetry, plots of NMR titration and variable-temperature NMR experiments, DOSY NMR spectra, traces of ITC experiments and a description of the computational experiments are provided in the Supplementary Information. Source data are provided with this paper.
References
Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004).
Georgakilas, V. et al. Functionalization of graphene: covalent and non-covalent approaches, derivatives and applications. Chem. Rev. 112, 6156–6214 (2012).
Watson, M. D., Jäckel, F., Severin, N., Rabe, J. P. & Müllen, K. A hexa-peri-hexabenzocoronene cyclophane: an addition to the toolbox for molecular electronics. J. Am. Chem. Soc. 126, 1402–1407 (2004).
Evans, P. J. et al. Synthesis of a helical bilayer nanographene. Angew. Chem. Int. Ed. 57, 6774–6779 (2018).
Zhao, X.-J. et al. Molecular bilayer graphene. Nat. Commun. 10, 3057 (2019).
Moshniaha, L. et al. Aromatic nanosandwich obtained by σ-dimerization of a nanographenoid π-radical. J. Am. Chem. Soc. 142, 3626–3635 (2020).
Young, R. M. & Wasielewski, M. R. Mixed electronic states in molecular dimers: connecting singlet fission, excimer formation and symmetry-breaking charge transfer. Acc. Chem. Res. 53, 1957–1968 (2020).
Zhang, Y. et al. Direct observation of a widely tunable bandgap in bilayer graphene. Nature 459, 820–823 (2009).
Lui, C. H., Li, Z., Mak, K. F., Cappelluti, E. & Heinz, T. F. Observation of an electrically tunable bandgap in trilayer graphene. Nat. Phys. 7, 944–947 (2011).
Cao, Y. et al. Tunable correlated states and spin-polarized phases in twisted bilayer–bilayer graphene. Nature 583, 215–220 (2020).
Matsumoto, I., Sekiya, R. & Haino, T. Self-assembly of nanographenes. Angew. Chem. Int. Ed. 60, 12706–12711 (2021).
Yoshizawa, M., Klosterman, J. K. & Fujita, M. Functional molecular flasks: new properties and reactions within discrete, self-assembled hosts. Angew. Chem. Int. Ed. 48, 3418–3438 (2009).
Juríček, M. et al. Induced-fit catalysis of corannulene bowl-to-bowl inversion. Nat. Chem. 6, 222–228 (2014).
Dale, E. J. et al. Supramolecular explorations: exhibiting the extent of extended cationic cyclophanes. Acc. Chem. Res. 49, 262–273 (2016).
Spenst, P. & Würthner, F. Photo- and redoxfunctional cyclophanes, macrocycles and catenanes based on aromatic bisimides. J. Photochem. Photobiol. C 31, 114–138 (2017).
Li, T. et al. Janusarene: a homoditopic molecular host. Angew. Chem. Int. Ed. 56, 9473–9477 (2017).
Xu, Y. & Delius, M. The supramolecular chemistry of strained carbon nanohoops. Angew. Chem. Int. Ed. 59, 559–573 (2020).
Benson, C. R. et al. Plug-and-play optical materials from fluorescent dyes and macrocycles. Chem 6, 1978–1997 (2020).
Narita, A., Wang, X.-Y., Feng, X. & Müllen, K. New advances in nanographene chemistry. Chem. Soc. Rev. 44, 6616–6643 (2015).
Stępień, M., Gońka, E., Żyła, M. & Sprutta, N. Heterocyclic nanographenes and other polycyclic heteroaromatic compounds: synthetic routes, properties and applications. Chem. Rev. 117, 3479–3716 (2017).
Ito, H., Segawa, Y., Murakami, K. & Itami, K. Polycyclic arene synthesis by annulative π-extension. J. Am. Chem. Soc. 141, 3–10 (2018).
Hirai, M., Tanaka, N., Sakai, M. & Yamaguchi, S. Structurally constrained boron-, nitrogen-, silicon- and phosphorus-centered polycyclic π-conjugated systems. Chem. Rev. 119, 8291–8331 (2019).
Liang, N., Meng, D. & Wang, Z. Giant rylene imide-based electron acceptors for organic photovoltaics. Acc. Chem. Res. 54, 961–975 (2021).
Seifert, S., Shoyama, K., Schmidt, D. & Würthner, F. An electron-poor C64 nanographene by palladium-catalyzed cascade C–C bond formation: one-pot synthesis and single-crystal structure analysis. Angew. Chem. Int. Ed. 55, 6390–6395 (2016).
Shoyama, K., Mahl, M., Seifert, S. & Würthner, F. A general synthetic route to polycyclic aromatic dicarboximides by palladium-catalyzed annulation reaction. J. Org. Chem. 83, 5339–5346 (2018).
Mahl, M., Shoyama, K., Krause, A. M., Schmidt, D. & Würthner, F. Base‐assisted imidization: a synthetic method for the introduction of bulky imide substituents to control packing and optical properties of naphthalene and perylene imides. Angew. Chem. Int. Ed. 59, 13401–13405 (2020).
Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 40, 1305–1323 (2011).
bindfit (Supramolecular, 2020); http://supramolecular.org
Dale, E. J. et al. ExCage. J. Am. Chem. Soc. 136, 10669–10682 (2014).
Mecozzi, S. & Rebek, J. Jr The 55% solution: a formula for molecular recognition in the liquid state. Chem. Eur. J. 4, 1016–1022 (1998).
Horn, P. R., Mao, Y. & Head-Gordon, M. Probing non-covalent interactions with a second generation energy decomposition analysis using absolutely localized molecular orbitals. Phys. Chem. Chem. Phys. 18, 23067–23079 (2016).
Cai, J. et al. Atomically precise bottom-up fabrication of graphene nanoribbons. Nature 466, 470–473 (2010).
Ciesielski, A. et al. Dynamic covalent chemistry of bisimines at the solid/liquid interface monitored by scanning tunnelling microscopy. Nat. Chem. 6, 1017–1023 (2014).
Feyter, S. D. & Schryver, F. C. D. Two-dimensional supramolecular self-assembly probed by scanning tunneling microscopy. Chem. Soc. Rev. 32, 139–150 (2003).
Zhao, Y. et al. The emergence of anion-π catalysis. Acc. Chem. Res. 51, 2255–2263 (2018).
Das, A. & Ghosh, S. Supramolecular assemblies by charge‐transfer interactions between donor and acceptor chromophores. Angew. Chem. Int. Ed. 53, 2038–2054 (2014).
Tayi, A. S. et al. Room-temperature ferroelectricity in supramolecular networks of charge-transfer complexes. Nature 488, 485–489 (2012).
Kang, S. J. et al. A supramolecular complex in small‐molecule solar cells based on contorted aromatic molecules. Angew. Chem. Int. Ed. 51, 8594–8597 (2012).
Zhang, J., Jin, J., Xu, H., Zhang, Q. & Huang, W. Recent progress on organic donor-acceptor complexes as active elements in organic field-effect transistors. J. Mater. Chem. C 6, 3485–3498 (2018).
Jiang, H. & Hu, W. The emergence of organic single‐crystal electronics. Angew. Chem. Int. Ed. 59, 1408–1428 (2019).
Sheldrick, G. M. SHELXT—integrated space-group and crystal-structure determination. Acta Crystallogr. A 71, 3–8 (2015).
Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. A 64, 112–122 (2008).
Spek, A. L. PLATON SQUEEZE: a tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. C 71, 9–18 (2015).
Spek, A. L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 36, 7–13 (2003).
Guzei, I. A. An idealized molecular geometry library for refinement of poorly behaved molecular fragments with constraints. J. Appl. Crystallogr. 47, 806–809 (2014).
Spek, A. L. Structure validation in chemical crystallography. Acta Crystallogr. D 65, 148–155 (2009).
Evans, P. R. & Murshudov, G. N. How good are my data and what is the resolution? Acta Crystallogr. D 69, 1204–1214 (2013).
Pracht, P., Bohle, F. & Grimme, S. Automated exploration of the low-energy chemical space with fast quantum chemical methods. Phys. Chem. Chem. Phys. 22, 7169–7192 (2020).
Spicher, S. & Grimme, S. Robust atomistic modeling of materials, organometallic and biochemical systems. Angew. Chem. Int. Ed. 59, 15665–15673 (2020).
Bannwarth, C., Ehlert, S. & Grimme, S. GFN2-xTB—an accurate and broadly parametrized self consistent tight-binding quantum chemical method with multipole electrostatics and density-dependent dispersion contributions. J. Chem. Theory Comput. 15, 1652–1671 (2019).
Frisch, M. J. et al. Gaussian 16, Revision A.03 (Gaussian, 2009).
Shao, Y. H. et al. Advances in molecular quantum chemistry contained in the Q-Chem 4 program package. Mol. Phys. 113, 184–215 (2015).
Acknowledgements
We thank the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) for financial support (grant no. WU 317/20-2).
Author information
Authors and Affiliations
Contributions
F.W. initiated and supervised the entire work. M.M. performed the synthesis and complexation experiments. M.M. and M.A.N. grew the single crystals for crystallographic analysis. K.S. conducted the crystallographic measurements and analysis. M.A.N. and K.S. conducted the DFT calculations. All authors contributed to writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Details of synthesis, crystallographic analysis and computation. Supplementary Figs. 1–22 and Tables 1–15.
Supplementary Data 1
Crystal structure of monolayer 1; CCDC 2068629.
Supplementary Data 2
Crystal structure of polylayer _(COR-1-COR)n; CCDC 2068630.
Supplementary Data 3
Crystal structure of hexalayer (COR-1-COR)2; CCDC 2068631.
Supplementary Data 4
Crystal structure of tetralayer COR-1-1-COR; CCDC 2068632.
Supplementary Source Data 1
Source Data Supplementary Figs. 7, 11a–d, 12a–c, 13b–d, 14a–c and 15. Fit of proton-signals from a 1H NMR titration experiment. Fit of UV–vis titration experiments of nanographene 1 in chloroform solutions. Fit of fluorescence titration experiments of nanographene 1 in chloroform solutions. Comparison of the average Gibbs free energies from UV–vis and fluorescence titration experiments with different guest molecules.
Source data
Source Data Fig. 4
Computed values of interaction energies from ALMO-EDA.
Rights and permissions
About this article
Cite this article
Mahl, M., Niyas, M.A., Shoyama, K. et al. Multilayer stacks of polycyclic aromatic hydrocarbons. Nat. Chem. 14, 457–462 (2022). https://doi.org/10.1038/s41557-021-00861-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00861-5