Abstract
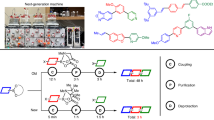
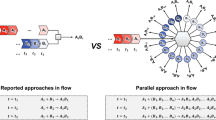
Recent advances in end-to-end continuous-flow synthesis are rapidly expanding the capabilities of automated customized syntheses of small-molecule pharmacophores, resulting in considerable industrial and societal impacts; however, many hurdles persist that limit the number of sequential steps that can be achieved in such systems, including solvent and reagent incompatibility between individual steps, cumulated by-product formation, risk of clogging and mismatch of timescales between steps in a processing chain. To address these limitations, herein we report a strategy that merges solid-phase synthesis and continuous-flow operation, enabling push-button automated multistep syntheses of active pharmaceutical ingredients. We demonstrate our platform with a six-step synthesis of prexasertib in 65% isolated yield after 32 h of continuous execution. As there are no interactions between individual synthetic steps in the sequence, the established chemical recipe file was directly adopted or slightly modified for the synthesis of twenty-three prexasertib derivatives, enabling both automated early and late-stage diversification.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information. A video of the SPS-flow automated synthesis of prexasertib is recorded as Supplementary Video 1.
Code availability
The LabVIEW code for operating the SPS-flow automated synthesis in this study is available at https://github.com/nus-automated-flow-system/auto-SPS-Flow-Supplementary-Software.
References
Cernak, T., Dykstra, K. D., Tyagarajan, S., Vachal, P. & Krska, S. W. The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 45, 546–576 (2016).
Moir, M., Danon, J. J., Reekie, T. A. & Kassiou, M. An overview of late-stage functionalization in today’s drug discovery. Expert Opin. Drug Discov. 14, 1137–1149 (2019).
Trobe, M. & Burke, M. D. The molecular industrial revolution: automated synthesis of small molecules. Angew. Chem. Int. Ed. 57, 4192–4214 (2018).
Ley, S. V., Fitzpatrick, D. E., Ingham, R. J. & Myers, R. M. Organic synthesis: march of the machines. Angew. Chem. Int. Ed. 54, 3449–3464 (2015).
Merrifield, R. B. Automated synthesis of peptides. Science 150, 178–185 (1965).
Alvarado-Urbina, G. et al. Automated synthesis of gene fragments. Science 214, 270–274 (1981).
Seeberger, P. H. & Werz, D. B. Automated synthesis of oligosaccharides as a basis for drug discovery. Nat. Rev. Drug Discov. 4, 751–763 (2005).
Li, J. et al. Synthesis of many different types of organic small molecules using one automated process. Science 347, 1221–1226 (2015).
Lehmann, J. W., Blair, D. J. & Burke, M. D. Towards the generalized iterative synthesis of small molecules. Nat. Rev. Chem. 2, 0115 (2018).
Woerly, E. M., Roy, J. & Burke, M. D. Synthesis of most polyene natural product motifs using just twelve building blocks and one coupling reaction. Nat. Chem. 6, 484–491 (2014).
Steiner, S. et al. Organic synthesis in a modular robotic system driven by a chemical programming language. Science 363, eaav2211 (2019).
Adamo, A. et al. On-demand continuous-flow production of pharmaceuticals in a compact, reconfigurable system. Science 352, 61–67 (2016).
Chatterjee, S., Guidi, M., Seeberger, P. H. & Gilmore, K. Automated radial synthesis of organic molecules. Nature 579, 379–384 (2020).
Hartman, R. L., McMullen, J. P. & Jensen, K. F. Deciding whether to go with the flow: evaluating the merits of flow reactors for synthesis. Angew. Chem. Int. Ed. 50, 7502–7519 (2011).
Gutmann, B., Cantillo, D. & Kappe, C. O. Continuous-flow technology—a tool for the safe manufacturing of active pharmaceutical ingredients. Angew. Chem. Int. Ed. 54, 6688–6728 (2015).
Snead, D. R. & Jamison, T. F. A three-minute synthesis and purification of ibuprofen: pushing the limits of continuous-flow processing. Angew. Chem. Int. Ed. 54, 983–987 (2015).
Lévesque, F. & Seeberger, P. H. Continuous-flow synthesis of the anti-malaria drug artemisinin. Angew. Chem. Int. Ed. 51, 1706–1709 (2012).
Tsubogo, T., Oyamada, H. & Kobayashi, S. Multistep continuous-flow synthesis of (R)- and (S)-rolipram using heterogeneous catalysts. Nature 520, 329–332 (2015).
Russell, M. G. & Jamison, T. F. Seven-step continuous flow synthesis of linezolid without intermediate purification. Angew. Chem. Int. Ed. 58, 7678–7681 (2019).
Sharma, M. K., Acharya, R. B., Shukla, C. A. & Kulkarni, A. A. Assessing the possibilities of designing a unified multistep continuous flow synthesis platform. Beilstein J. Org. Chem. 14, 1917–1936 (2018).
Bana, P. et al. The route from problem to solution in multistep continuous flow synthesis of pharmaceutical compounds. Bioorg. Med. Chem. 25, 6180–6189 (2017).
Baxendale, I. R. et al. A flow process for the multi-step synthesis of the alkaloid natural product oxomaritidine: a new paradigm for molecular assembly. Chem. Commun. 2566–2568 (2006).
Ley, S. V. On being green: can flow chemistry help? Chem. Rec. 12, 378–390 (2012).
Merrifield, B. Solid phase synthesis. Science 232, 341–347 (1986).
Guillier, F., Orain, D. & Bradley, M. Linkers and cleavage strategies in solid-phase organic synthesis and combinatorial chemistry. Chem. Rev. 100, 2091–2158 (2000).
Palmieri, A., Ley, S. V., Polyzos, A., Ladlow, M. & Baxendale, I. R. Continuous flow based catch and release protocol for the synthesis of α-ketoesters. Beilstein J. Org. Chem. 5, 23 (2009).
Baxendale, I. R., Ley, S. V., Smith, C. D. & Tranmer, G. K. A flow reactor process for the synthesis of peptides utilizing immobilized reagents, scavengers and catch and release protocols. Chem. Commun. 4835–4837 (2006).
Hopkin, M. D., Baxendale, I. R. & Ley, S. V. A flow-based synthesis of imatinib: the API of Gleevec. Chem. Commun. 46, 2450–2452 (2010).
Nicolaou, K. C. et al. Synthesis of epothilones A and B in solid and solution phase. Nature 387, 268–272 (1997).
Nandy, J. P. et al. Advances in solution- and solid-phase synthesis toward the generation of natural product-like libraries. Chem. Rev. 109, 1999–2060 (2009).
Plante, O. J., Palmacci, E. R. & Seeberger, P. H. Automated solid-phase synthesis of oligosaccharides. Science 291, 1523–1527 (2001).
Mijalis, A. J. et al. A fully automated flow-based approach for accelerated peptide synthesis. Nat. Chem. Biol. 13, 464–466 (2017).
Njarðarson Group. Top 200 small molecule pharmaceuticals by retail sales in 2018. The University of Arizona https://njardarson.lab.arizona.edu/sites/njardarson.lab.arizona.edu/files/Top%20200%20Small%20Molecule%20Pharmaceuticals%202018V4.pdf (2018).
Coley, C. W. et al. A robotic platform for flow synthesis of organic compounds informed by AI planning. Science 365, eaax1566 (2019).
Bédard, A. C. et al. Reconfigurable system for automated optimization of diverse chemical reactions. Science 361, 1220–1225 (2018).
Cole, K. P. et al. Kilogram-scale prexasertib monolactate monohydrate synthesis under continuous-flow CGMP conditions. Science 356, 1144–1150 (2017).
Farouz, F. S., Holcomb, R. C., Kasar, R. & Myers, S. S. Compounds useful for inhibiting chk1. WO Patent 2010077758 A1 (2010).
Bagley, M. C. et al. The effect of RO3201195 and a pyrazolyl ketone P38 MAPK inhibitor library on the proliferation of Werner syndrome cells. Org. Biomol. Chem. 14, 947–956 (2016).
Baron, H., Remfry, F. G. P. & Thorpe, J. F. The formation and reactions of imino-compounds. Part I. Condensation of ethyl cyanoacetate with its sodium derivative. J. Chem. Soc. Trans. 85, 1726–1761 (1904).
Munirathinam, R., Huskens, J. & Verboom, W. Supported catalysis in continuous-flow microreactors. Adv. Synth. Catal. 357, 1093–1123 (2015).
Schneider, G. & Fechner, U. Computer-based de novo design of drug-like molecules. Nat. Rev. Drug Discov. 4, 649–663 (2005).
Scott, P. J. H. Linker Strategies in Solid-Phase Organic Synthesis (John Wiley & Sons, 2009)
Blaney, P., Grigg, R. & Sridharan, V. Traceless solid-phase organic synthesis. Chem. Rev. 102, 2607–2624 (2002).
Cankařová, N., Schütznerová, E. & Krchňák, V. Traceless solid-phase organic synthesis. Chem. Rev. 119, 12089–12207 (2019).
Sanghvi, Y. S. A status update of modified oligonucleotides for chemotherapeutics applications. Curr. Protoc. Nucl. Acid Chem. 46, 4.1.1–4.1.22 (2011).
Schneider, G. Automating drug discovery. Nat. Rev. Drug Discov. 17, 97–113 (2018).
Acknowledgements
This work was supported by the Agency for Science, Technology and Research (A*STAR) of Singapore RIE2020 AME IRG (grant no. A1783c0013). We thank J. T. Njardarson for allowing us to reproduce and modify the poster ‘Top 200 small molecule pharmaceuticals by retail sales in 2018’ (see Supplementary Table 11).
Author information
Authors and Affiliations
Contributions
J.W. conceived and designed the experiments. C.L., W.W., M.W. and W.C. performed the experiments. J.W., S.A.K., C.L. and M.W. analysed the experimental data. C.L. and J.R. built the SPS-flow reactor system. J.X. performed the programming for automated control. B.I.S. and L.-W.D. performed the preliminary biological screening. J.W. and S.A.K. wrote the manuscript with input from all authors. All of the authors have approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.W., S.A.K., C.L., W.W., W.C., M.W. and J.X. are inventors on International Patent Application PCT/SG2020/050603 filed by the National University of Singapore, which covers the synthesis of non-peptide small-molecules using the strategy of automated SPS-flow synthesis. The authors declare no other competing interests.
Additional information
Peer review information Nature Chemistry thanks Kevin Cole, Christopher Gordon and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information
Supplementary Figs. 1–64, Tables 1–11, synthetic procedural details, automation development and implementation, high-resolution mass spectrometry, infrared data, NMR data and spectra, HPLC spectra.
Supplementary Video 1
Recording of the automated synthesis of prexasertib using the SPS-flow system.
Supplementary Data 1
Raw data for the bioactivity evaluation shown in Supplementary Fig. 23.
Rights and permissions
About this article
Cite this article
Liu, C., Xie, J., Wu, W. et al. Automated synthesis of prexasertib and derivatives enabled by continuous-flow solid-phase synthesis. Nat. Chem. 13, 451–457 (2021). https://doi.org/10.1038/s41557-021-00662-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00662-w