Abstract
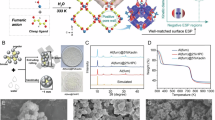
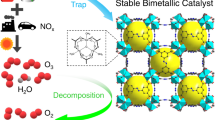
Air pollution by nitrogen oxides, NOx, is a major problem, and new capture and abatement technologies are urgently required. Here, we report a metal–organic framework (Manchester Framework Material 520 (MFM-520)) that can efficiently confine dimers of NO2, which results in a high adsorption capacity of 4.2 mmol g–1 (298 K, 0.01 bar) with full reversibility and no loss of capacity over 125 cycles. Treatment of NO2@MFM-520 with water in air leads to a quantitative conversion of the captured NO2 into HNO3, an important feedstock for fertilizer production, and fully regenerates MFM-520. The confinement of N2O4 inside nanopores was established at a molecular level, and the dynamic breakthrough experiments using both dry and humid NO2 gas streams verify the excellent stability and selectivity of MFM-520 and confirm its potential for precious-metal-free deNOx technologies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre under deposition numbers CCDC 1556634 (bare MOF, 298 K), 1556637 (NO2-loaded MOF, 273 K), 1556636 (NO2-loaded MOF, 283 K), 1556635 (NO2-loaded MOF, 298 K), 1556638 (NO2-loaded MOF, 313 K), 1556639 (NO2-loaded MOF, 333 K), 1556640 (NO2-loaded MOF, 353 K), 1556641 (NO2-loaded MOF, 373 K) and 1556642 (regenerated MOF, 393 K). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. All the other relevant data that support the findings of this study are available within the article and its Supplementary Information, or from the corresponding author upon reasonable request.
References
Amann, M., Klimont, Z. & Wagner, F. Regional and global emissions of air pollutants: recent trends and future scenarios. Annu. Rev. Environ. Resour. 38, 31–55 (2013).
Wall, D. H., Nielsen, U. N. & Six, J. Soil biodiversity and human health. Nature 528, 69–76 (2015).
Edwards, P. M. et al. High winter ozone pollution from carbonyl photolysis in an oil and gas basin. Nature 514, 351–354 (2014).
Chen, Z., Wang, J., Ma, G. & Zhang, Y. China tackles the health effects of air pollution. Lancet 382, 1959–1960 (2013).
Mladenovic, M., Paprika, M. & Marinkovic, A. Denitrification techniques for biomass combustion. Renew. Sustain. Energy Rev. 82, 3350–3364 (2018).
Park, J. H., Ahn, J. W., Kim, K. H. & Son, Y. S. History and futuristic review of electron beam technology for the treatment of SO2 and NOx in flue gas. Chem. Eng. J. 355, 351–366 (2019).
Selleri, T., Nova, I., Tronconi, E., Schmeisser, V. & Seher, S. The impact of light and heavy hydrocarbons on the NH3-SCR activity of commercial Cu- and Fe-zeolite catalysts. Catal. Today 320, 100–111 (2019).
Ambient (outdoor) air quality and health. WHO www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (2018).
Han, X., Yang, S. & Schröder, M. Porous metal–organic framework as emerging sorbents for clean air. Nat. Rev. Chem. 3, 108–118 (2019).
Chen, J. et al. Beyond fossil fuel-driven nitrogen transformations. Science 360, eaar6611 (2018).
Breedon, M., Spencer, M. J. S. & Miura, N. Adsorption of NO2 on YSZ(111) and oxygen-enriched YSZ(111) surfaces. J. Phys. Chem. C 117, 12472–12482 (2013).
Shirahama, N. et al. Mechanistic study on adsorption and reduction of NO2 over activated carbon fibers. Carbon 40, 2605–2611 (2002).
Wang, X., Hanson, J. C., Kwak, J. H., Szanyi, J. & Peden, C. H. Cation movements during dehydration and NO2 desorption in a Ba–Y, FAU zeolite: an in situ time-resolved X-ray diffraction study. J. Phys. Chem. C 117, 3915–3922 (2013).
Zhou, X. et al. Capture of pure toxic gases through porous materials from molecular simulations. Mol. Phys. 116, 2095–2107 (2018).
Furukawa, H., Cordova, K. E., O’Keefe, M. & Yaghi, O. M. The chemistry and applications of metal–organic frameworks. Science 341, 1230444 (2013).
Adil, K. et al. Gas/vapour separation using ultra-microporous metal–organic frameworks: insights into the structure/separation relationship. Chem. Soc. Rev. 46, 3402–3430 (2017).
Han, X. et al. Reversible adsorption of nitrogen dioxide within a robust porous metal–organic framework. Nat. Mater. 17, 691–696 (2018).
Seddiek, I. S. & Elgohary, M. M. Eco-friendly selection of ship emissions reduction strategies with emphasis on SOx and NOx emissions. Int. J. Nav. Archit. Ocean Eng. 6, 737–748 (2014).
Ebrahim, A. M. & Bandosz, T. J. Effect of amine modification on the properties of zirconium–carboxylic acid based materials and their applications as NO2 adsorbents at ambient conditions. Microporous Mesoporous Mater. 188, 149–162 (2014).
Ebrahim, A. M. & Bandosz, T. J. Ce(iii) doped Zr-based MOFs as excellent NO2 adsorbents at ambient conditions. ACS Appl. Mater. Interfaces 5, 10565–10573 (2013).
Levasseur, B., Petit, C. & Bandosz, J. T. Reactive adsorption of NO2 on copper-based metal–organic framework and graphite oxide/metal–organic framework composites. ACS Appl. Mater. Interfaces 2, 3606–3613 (2010).
Petit, C., Levasseur, B., Mendoza, B. & Bandosz, J. T. Reactive adsorption of acidic gases on MOF/graphite oxide composites. Microporous Mesoporous Mater. 154, 107–112 (2012).
Lin, X. et al. A porous framework polymer based on a zinc(ii) 4,4′-bipyridine-2,6,2′,6′-tetracarboxylate: synthesis, structure, and ‘zeolite-like’ behaviors. J. Am. Chem. Soc. 128, 10745–10753 (2006).
Myers, A. L. & Prausnitz, J. M. Thermodynamics of mixed-gas adsorption. AlChE J. 11, 121–127 (1965).
Cartwright, S. B. & Robertson, H. J. N2O4: change of dimensions with temperature. Chem. Commun. 82–83 (1966).
Nagata, M., Yahiro, H., Shiotani, M., Lindgren, M. & Lund, A. ESR study of the motional dynamics of NO2 adsorbed on Na-mordenite. Chem. Phys. Lett. 256, 27–32 (1996).
Vosper, J. A. Dissociation of dinitrogen tetroxide in the gas phase. J. Chem. Soc. A 1970, 625–627 (1970).
Vosper, J. A. Dissociation of dinitrogen tetroxide in the liquid phase. J. Chem. Soc. A 1970, 2191–2193 (1970).
Kato, T., Hayashi, S. & Machida, K. Molecular dynamics simulation of liquid N2O4 ⇌ 2NO2 by orientation-sensitive pairwise potential. I. Chemical equilibrium. J. Chem. Phys. 115, 10852–10862 (2001).
Matito-Martos, I. et al. Adsorption equilibrium of nitrogen dioxide in porous materials. Phys. Chem. Chem. Phys. 20, 4189–4199 (2018).
Acknowledgements
We thank EPSRC (EP/I011870, EP/P001386, EP/K038869), ERC (AdG 742041) and the Royal Society and University of Manchester for funding, and EPSRC for funding of the EPSRC National EPR Facility at Manchester. We are especially grateful to the Advanced Light Source (ALS) and Oak Ridge National Laboratory (ORNL) for access to the beamline 11.3.1 and VISION, respectively. This research used resources of the Advanced Light Source, which is a DOE Office of Science User Facility under contract no. DE-AC02-05CH11231. Computing resources were made available through the VirtuES and the ICE-MAN projects, funded by the Laboratory Directed Research and Development program at ORNL. J.L. and X.Z. thank the China Scholarship Council for funding, and A.M.S. thanks the Russian Science Foundation (grant no. 17-73-10320) and the Royal Society of Chemistry for funding. We also thank M. A. Denecke for helpful discussions.
Author information
Authors and Affiliations
Contributions
J.L. synthesized and characterized the MOF samples, and measured the adsorption isotherms. J.L. and X.H. measured and analysed the breakthrough data. S.Y., X.Z., J.L., S.J.T. and L.J.M.M. collected and analysed the in situ synchrotron X-ray diffraction data. Y.C., L.L.D. and A.J.R.-C. collected and analysed the molecular dynamics MD modelling and neutron scattering data. J.L., X.H., A.M.S., F.T. and E.J.L.M. collected and analysed the EPR data. M.S. and S.Y. directed and supervised the project and prepared the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, Figs. 1–21, Tables 1–5 and refs 1–12.
Crystallographic data
CIF for bare MOF, 298 K; CCDC reference: 1556634.
Crystallographic data
CIF for NO2-loaded MOF, 273 K; CCDC reference: 1556637.
Crystallographic data
CIF for NO2-loaded MOF, 283 K; CCDC reference: 1556636.
Crystallographic data
CIF for NO2-loaded MOF, 298 K; CCDC reference: 1556635.
Crystallographic data
CIF for NO2-loaded MOF, 313 K; CCDC reference: 1556638.
Crystallographic data
CIF for NO2-loaded MOF, 333 K; CCDC reference: 1556639.
Crystallographic data
CIF for NO2-loaded MOF, 353 K; CCDC reference: 1556640.
Crystallographic data
CIF for NO2-loaded MOF, 373 K; CCDC reference: 1556641.
Crystallographic data
CIF for regenerated MOF, 393 K; CCDC reference: 1556642.
Rights and permissions
About this article
Cite this article
Li, J., Han, X., Zhang, X. et al. Capture of nitrogen dioxide and conversion to nitric acid in a porous metal–organic framework. Nat. Chem. 11, 1085–1090 (2019). https://doi.org/10.1038/s41557-019-0356-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-019-0356-0