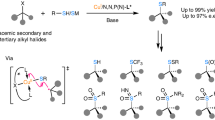
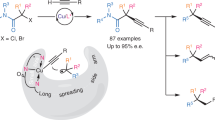
Abstract
Continued development of the Sonogashira coupling has made it a well established and versatile reaction for the straightforward formation of C–C bonds, forging the carbon skeletons of broadly useful functionalized molecules. However, asymmetric Sonogashira coupling, particularly for C(sp3)–C(sp) bond formation, has remained largely unexplored. Here we demonstrate a general stereoconvergent Sonogashira C(sp3)–C(sp) cross-coupling of a broad range of terminal alkynes and racemic alkyl halides (>120 examples) that are enabled by copper-catalysed radical-involved alkynylation using a chiral cinchona alkaloid-based P,N-ligand. Industrially relevant acetylene and propyne are successfully incorporated, laying the foundation for scalable and economic synthetic applications. The potential utility of this method is demonstrated in the facile synthesis of stereoenriched bioactive or functional molecule derivatives, medicinal compounds and natural products that feature a range of chiral C(sp3)–C(sp/sp2/sp3) bonds. This work emphasizes the importance of radical species for developing enantioconvergent transformations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All of the characterization data and experimental protocols are provided in this article and its Supplementary Information. Data are also available from the corresponding author on request.
References
Knochel, P. & Molander, G. A. Comprehensive Organic Synthesis 2nd edn (Elsevier, Amsterdam, 2014).
de Meijere, A., Bräse, S. & Oestreich, M. Metal-Catalyzed Cross-Coupling Reactions and More (Wiley-VCH, Weinheim, 2014).
Girard, S. A., Knauber, T. & Li, C.-J. The cross-dehydrogenative coupling of Csp 3–H bonds: a versatile strategy for C–C bond formations. Angew. Chem. Int. Ed. 53, 74–100 (2014).
Trost, B. M. & Li, C.-J. Modern Alkyne Chemistry: Catalytic and Atom-Economic Transformations (Wiley-VCH, Weinheim, 2015).
Dieck, H. A. & Heck, F. R. Palladium catalyzed synthesis of aryl, heterocyclic and vinylic acetylene derivatives. J. Organomet. Chem. 93, 259–263 (1975).
Cassar, L. Synthesis of aryl- and vinyl-substituted acetylene derivatives by the use of nickel and palladium complexes. J. Organomet. Chem. 93, 253–257 (1975).
Sonogashira, K., Tohda, Y. & Hagihara, N. A convenient synthesis of acetylenes: catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett. 16, 4467–4470 (1975).
Chinchilla, R. & Nájera, C. The Sonogashira reaction: a booming methodology in synthetic organic chemistry. Chem. Rev. 107, 874–922 (2007).
Eckhardt, M. & Fu, G. C. The first applications of carbene ligands in cross-couplings of alkyl electrophiles: Sonogashira reactions of unactivated alkyl bromides and iodides. J. Am. Chem. Soc. 125, 13642–13643 (2003).
Altenhoff, G., Würtz, S. & Glorius, F. The first palladium-catalyzed Sonogashira coupling of unactivated secondary alkyl bromides. Tetrahedron Lett. 47, 2925–2928 (2006).
Hornillos, V. et al. Synthesis of axially chiral heterobiaryl alkynes via dynamic kinetic asymmetric alkynylation. Chem. Commun. 52, 14121–14124 (2016).
Cui, X.-Y. et al. (Guanidine)copper complex-catalyzed enantioselective dynamic kinetic allylic alkynylation under biphasic condition. J. Am. Chem. Soc. 140, 8448–8455 (2018).
Harada, A., Makida, Y., Sato, T., Ohmiya, H. & Sawamura, M. Copper-catalyzed enantioselective allylic alkylation of terminal alkyne pronucleophiles. J. Am. Chem. Soc. 136, 13932–13939 (2014).
Hamilton, J. Y., Sarlah, D. & Carreira, E. M. Iridium-catalyzed enantioselective allylic alkynylation. Angew. Chem. Int. Ed. 52, 7532–7535 (2013).
Dabrowski, J. A., Gao, F. & Hoveyda, A. H. Enantioselective synthesis of alkyne-substituted quaternary carbon stereogenic centers through NHC–Cu-catalyzed allylic substitution reactions with (i-Bu)2(alkynyl)aluminum reagents. J. Am. Chem. Soc. 133, 4778–4781 (2011).
Choi, J. & Fu, G. C. Transition metal–catalyzed alkyl-alkyl bond formation: another dimension in cross-coupling chemistry. Science 356, eaaf7230 (2017).
Fu, G. C. Transition-metal catalysis of nucleophilic substitution reactions: a radical alternative to SN1 and SN2 processes. ACS Cent. Sci. 3, 692–700 (2017).
Cherney, A. H., Kadunce, N. T. & Reisman, S. E. Enantioselective and enantiospecific transition-metal-catalyzed cross-coupling reactions of organometallic reagents to construct C–C bonds. Chem. Rev. 115, 9587–9652 (2015).
Hazra, A., Lee, M. T., Chiu, J. F. & Lalic, G. Photoinduced copper-catalyzed coupling of terminal alkynes and alkyl iodides. Angew. Chem. Int. Ed. 57, 5492–5496 (2018).
Voronin, V. V., Ledovskaya, M. S., Bogachenkov, A. S., Rodygin, K. S. & Ananikov, V. P. Acetylene in organic synthesis: recent progress and new uses. Molecules 23, E2442 (2018).
Trotus, I.-T., Zimmermann, T. & Schüth, F. Catalytic reactions of acetylene: a feedstock for the chemical industry revisited. Chem. Rev. 114, 1761–1782 (2014).
Sasaki, H., Boyall, D. & Carreira, E. M. Facile, asymmetric addition of acetylene to aldehydes: in situ generation of reactive zinc acetylide. Helv. Chim. Acta 84, 964–971 (2001).
Sato, Y., Nishimata, T. & Mori, M. Asymmetric synthesis of isoindoline and isoquinoline derivatives using nickel(0)-catalyzed [2+2+2] cocyclization. J. Org. Chem. 59, 6133–6135 (1994).
Shibata, T., Arai, Y. & Tahara, Y.-k Enantioselective construction of quaternary carbon centers by catalytic [2+2+2] cycloaddition of 1,6-enynes and alkynes. Org. Lett 7, 4955–4957 (2005).
Kong, J. R. & Krische, M. J. Catalytic carbonyl Z-dienylation via multicomponent reductive coupling of acetylene to aldehydes and α-ketoesters mediated by hydrogen: carbonyl insertion into cationic rhodacyclopentadienes. J. Am. Chem. Soc. 128, 16040–16041 (2006).
Heller, B. et al. Phosphorus-bearing axially chiral biaryls by catalytic asymmetric cross-cyclotrimerization and a first application in asymmetric hydrosilylation. Chem. Eur. J. 13, 1117–1128 (2007).
Skucas, E., Kong, J. R. & Krische, M. J. Enantioselective reductive coupling of acetylene to N-arylsulfonyl imines via rhodium catalyzed C–C bond-forming hydrogenation: (Z)-dienyl allylic amines. J. Am. Chem. Soc. 129, 7242–7243 (2007).
Evano, G. & Blanchard, N. Copper-Mediated Cross-Coupling Reactions (Wiley, New Jersey, 2014).
Shaughnessy, K. H., Ciganek, E. & DeVasher, R. B. Copper-Catalyzed Amination of Aryl and Alkenyl Electrophiles (Wiley, New Jersey, 2017).
Díez-González, S. in Advances in Organometallic Chemistry (ed. Pérez, P. J.) 93–141 (Academic, 2016).
Kainz, Q. M. et al. Asymmetric copper-catalyzed C–N cross-couplings induced by visible light. Science 351, 681–684 (2016).
Yoshikai, N. & Nakamura, E. Mechanisms of nucleophilic organocopper(i) reactions. Chem. Rev. 112, 2339–2372 (2012).
Pérez García, P. M., Ren, P., Scopelliti, R. & Hu, X. Nickel-catalyzed direct alkylation of terminal alkynes at room temperature: a hemilabile pincer ligand enhances catalytic activity. ACS Catal. 5, 1164–1171 (2015).
Yi, J., Lu, X., Sun, Y.-Y., Xiao, B. & Liu, L. Nickel-catalyzed Sonogashira reactions of non-activated secondary alkyl bromides and iodides. Angew. Chem. Int. Ed. 52, 12409–12413 (2013).
Vechorkin, O., Barmaz, D., Proust, V. & Hu, X. Ni-catalyzed Sonogashira coupling of nonactivated alkyl halides: orthogonal functionalization of alkyl iodides, bromides, and chlorides. J. Am. Chem. Soc. 131, 12078–12079 (2009).
Wang, Z. et al. Sonogashira reactions of alkyl halides catalyzed by NHC [CNN] pincer nickel(ii) complexes. New J. Chem. 42, 11465–11470 (2018).
Luo, F.-X. et al. Cu-catalyzed alkynylation of unactivated C(sp 3)–X bonds with terminal alkynes through directing strategy. Org. Lett. 18, 2040–2043 (2016).
Fantin, M., Lorandi, F., Gennaro, A., Isse, A. A. & Matyjaszewski, K. Electron transfer reactions in atom transfer radical polymerization. Synthesis 49, 3311–3322 (2017).
Leophairatana, P., Samanta, S., De Silva, C. C. & Koberstein, J. T. Preventing alkyne–alkyne (i.e., Glaser) coupling associated with the ATRP synthesis of alkyne-functional polymers/macromonomers and for alkynes under click (i.e., CuAAC) reaction conditions. J. Am. Chem. Soc. 139, 3756–3766 (2017).
Kolb, H. C., VanNieuwenhze, M. S. & Sharpless, K. B. Catalytic asymmetric dihydroxylation. Chem. Rev. 94, 2483–2547 (1994).
Sladojevich, F., Trabocchi, A., Guarna, A. & Dixon, D. J. A new family of cinchona-derived amino phosphine precatalysts: application to the highly enantio- and diastereoselective silver-catalyzed isocyanoacetate aldol reaction. J. Am. Chem. Soc. 133, 1710–1713 (2011).
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014).
Berliner, M. A., Cordi, E. M., Dunetz, J. R. & Price, K. E. Sonogashira reactions with propyne: facile synthesis of 4-hydroxy-2-methylbenzofurans from iodoresorcinols. Org. Process Res. Dev. 14, 180–187 (2010).
Schobert, H. Production of acetylene and acetylene-based chemicals from coal. Chem. Rev. 114, 1743–1760 (2014).
John, J. Global acetylene gas market will expand revenue USD 6 Bn by 2020. SBWire (15 March 2015).
Schwarzwalder, G. M., Matier, C. D. & Fu, G. C. Enantioconvergent cross-couplings of alkyl electrophiles: the catalytic asymmetric synthesis of organosilanes. Angew. Chem. Int. Ed. 58, 3571–3574 (2019).
Meanwell, N. A. Synopsis of some recent tactical application of bioisosteres in drug design. J. Med. Chem. 54, 2529–2591 (2011).
Armstrong, M. K., Goodstein, M. B. & Lalic, G. Diastereodivergent reductive cross coupling of alkynes through tandem catalysis: Z- and E-selective hydroarylation of terminal alkynes. J. Am. Chem. Soc. 140, 10233–10241 (2018).
Elford, T. G., Nave, S., Sonawane, R. P. & Aggarwal, V. K. Total synthesis of (+)-erogorgiaene using lithiation–borylation methodology, and stereoselective synthesis of each of its diastereoisomers. J. Am. Chem. Soc. 133, 16798–16801 (2011).
Wu, L. et al. Asymmetric synthesis of (R)-ar-curcumene, (R)-4,7-dimethyl-l-tetralone, and their enantiomers via cobalt-catalyzed asymmetric Kumada cross-coupling. Tetrahedron: Asymmetry 27, 78–83 (2016).
Bianco, G. G. et al. (+)- and (−)-Mutisianthol: first total synthesis, absolute configuration, and antitumor activity. J. Org. Chem. 74, 2561–2566 (2009).
Kamal, A., Shaheer Malik, M., Azeeza, S., Bajee, S. & Shaik, A. A. Total synthesis of (R)- and (S)-turmerone and (7S,9R)-bisacumol by an efficient chemoenzymatic approach. Tetrahedron: Asymmetry 20, 1267–1271 (2009).
Schaub, T. A. & Kivala, M. in Metal-Catalyzed Cross-Coupling Reactions and More (eds de Meijere, A., Bräse, S. & Oestreich, M.) 665–762 (Wiley-VCH, 2014).
Zuidema, E. & Bolm, C. Sub-mol% catalyst loading and ligand-acceleration in the copper-catalyzed coupling of aryl iodides and terminal alkyenes. Chem. Eur. J. 16, 4181–4185 (2010).
Acknowledgements
Financial support for this work was provided by the National Natural Science Foundation of China (grant nos. 21722203, 21831002 and 21801116), Shenzhen Special Funds (grant nos. JCYJ20170412152435366 and JCYJ20170307105638498) and Shenzhen Nobel Prize Scientists Laboratory Project (grant no. C17783101).
Author information
Authors and Affiliations
Contributions
X.-Y.D., Y.-F.Z., C.-L.M., Q.-S.G. and F.-L.W. designed the experiments and analysed the data. X.-Y.D., Y.-F.Z., C.-L.M., F.-L.W., Z.-L.L. and S.-P.J. performed the experiments. All authors participated in writing the manuscript. X.-Y.L. conceived and supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Characterization data, experimental data, synthetic procedures, figures and tables.
Rights and permissions
About this article
Cite this article
Dong, XY., Zhang, YF., Ma, CL. et al. A general asymmetric copper-catalysed Sonogashira C(sp3)–C(sp) coupling. Nat. Chem. 11, 1158–1166 (2019). https://doi.org/10.1038/s41557-019-0346-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-019-0346-2