Abstract
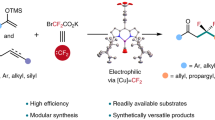
Difluorocarbene has important applications in pharmaceuticals, agrochemicals and materials, but all these applications proceed using just a few types of reaction by taking advantage of its intrinsic electrophilicity. Here, we report a palladium-catalysed strategy that confers the formed palladium difluorocarbene (Pd=CF2) species with both nucleophilicity and electrophilicity by switching the valence state of the palladium centre (Pd(0) and Pd(ii), respectively). Controllable catalytic difluorocarbene transfer occurs between readily available arylboronic acids and the difluorocarbene precursor diethyl bromodifluoromethylphosphonate (BrCF2PO(OEt)2). From just this simple fluorine source, difluorocarbene transfer enables access to four types of product: difluoromethylated and tetrafluoroethylated arenes and their corresponding fluoroalkylated ketones. The transfer can also be applied to the modification of pharmaceuticals and agrochemicals as well as the one-pot diversified synthesis of fluorinated compounds. Mechanistic and computational studies consistently reveal that competition between nucleophilic and electrophilic palladium difluorocarbene ([Pd]=CF2) is the key factor controlling the catalytic difluorocarbene transfer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Center under deposition numbers CCDC 1902891 (B1-1), 1916606 (B1-2), 1916607 (A1-2), 1902894 (C1-1a), 1902898 (C2), 1902901 (E1) and 1902900 (cis-G1). Copies of the data can be obtained free of charge from https://www.ccdc.cam.ac.uk/strucutres/. All other data supporting the findings of this study are available within the Article and its Supplementary Information, or from the corresponding author upon reasonable request.
References
Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications 2nd edn (Wiley-VCH, 2013).
Brahms, D. L. S. & Dailey, W. P. Fluorinated carbenes. Chem. Rev. 96, 1585–1632 (1996).
Ni, C. & Hu, J. Recent advances in the synthetic application of difluorocarbene. Synthesis 46, 842–863 (2014).
Hudlicky, M. & Pavlath, A. E. Chemistry of Organic Fluorine Compounds II (American Chemical Society, 1995).
Miller, T. G. & Thanassi, J. W. The preparation of aryl difluoromethyl ethers. J. Org. Chem. 25, 2009–2012 (1960).
Hohlfeld, J. M. et al. Roflumilast attenuates pulmonary inflammation upon segmental endotoxin challenge in healthy subjects: a randomized placebo-controlled trial. Pulm. Pharmacol. Ther. 21, 616–623 (2008).
Dolbier, W. R. Jr. & Battiste, M. A. Structure, synthesis and chemical reactions of fluorinated cyclopropanes and cyclopropenes. Chem. Rev. 103, 1071–1098 (2003).
Burch, J. D. et al. Tetrahydroindazoles as interleukin-2 inducible T-cell kinase inhibitors. Part II. Second-generation analogues with enhanced potency, selectivity and pharmacodynamic modulation in vivo. J. Med. Chem. 58, 3806–3816 (2015).
Fuqua, S. A., Duncan, W. G. & Silverstein, R. M. A one-step synthesis of 1,1-difluoroolefins from aldehydes by a modified Wittig synthesis. Tetrahedron Lett. 5, 1461–1463 (1964).
MacNeil, J. G.Jr & Burton, D. J. Generation of trifluoromethylcopper from chlorodifluoroacetate. J. Fluor. Chem. 55, 225–227 (1991).
Duan, J.-X., Su, D.-B. & Chen, Q.-Y. Trifluoromethylation of organic halides with methyl halodifluoroacetates—a process via difluorocarbene and trifluoromethide intermediates. J. Fluor. Chem. 61, 279–284 (1993).
Huiban, M. et al. A broadly applicable [18F]trifluoromethylation of aryl and heteroaryl iodides for PET imaging. Nat. Chem. 5, 941–944 (2013).
Levin, M. D. et al. A catalytic fluoride-rebound mechanism for C(sp 3)–CF3 bond formation. Science 356, 1272–1276 (2017).
Dilman, A. D. & Levin, V. V. Difluorocarbene as a building block for consecutive bond-forming reactions. Acc. Chem. Res. 51, 1272–1280 (2018).
Reger, D. L. & Dukes, M. D. Molybdenum perfluorocarbene complexes. J. Organomet. Chem. 153, 67–72 (1978).
Brothers, P. J. & Roper, W. R. Transition-metal dihalocarbene complexes. Chem. Rev. 88, 1293–1326 (1988).
Harrison, D. J., Gorelsky, S. I., Lee, G. M., Korobkov, I. & Baker, R. T. Cobalt fluorocarbene complexes. Organometallics 32, 12–15 (2013).
Harrison, D. J., Daniels, A. L., Korobkov, I. & Baker, R. T. d 10 nickel difluorocarbenes and their cycloaddition reactions with tetrafluoroethylene. Organometallics 34, 5683–5686 (2015).
Trnka, T. M., Day, M. W. & Grubbs, R. H. Olefin metathesis with 1,1-difluoroethylene. Angew. Chem. Int. Ed. 40, 3441–3444 (2001).
Takahira, Y. & Morizawa, Y. Ruthenium-catalyzed olefin cross-metathesis with tetrafluoroethylene and analogous fluoroolefins. J. Am. Chem. Soc. 137, 7031–7034 (2015).
Feng, Z., Min, Q.-Q. & Zhang, X. Access to difluoromethylated arenes by Pd-catalyzed reaction of arylboronic acids with bromodifluoroacetate. Org. Lett. 18, 44–47 (2016).
Feng, Z., Min, Q.-Q., Fu, X.-P., An, L. & Zhang, X. Chlorodifluoromethane-triggered formation of difluoromethylated arenes catalysed by palladium. Nat. Chem. 9, 918–923 (2017).
Deng, X.-Y., Lin, J.-H. & Xiao, J.-C. Pd-catalyzed transfer of difluorocarbene. Org. Lett. 18, 4384–4387 (2016).
Clark, G. R., Hoskins, S. V., Jones, T. C. & Roper, W. R. Oxidation state control of the reactivity of a transition metal–carbon double bond. Synthesis, X-ray crystal structure, and reactions of the zerovalent difluorocarbene complex [Ru(=CF2)(CO)2(PPh3)2]. J. Chem. Soc. Chem. Commun. 719–721 (1983).
Brothers, P. J., Burrell, A. K., Clark, G. R., Rickard, C. E. F. & Roper, W. R. Trifluoromethyl, difluorocarbene and tetrafluoroethylene complexes of iridium and the crystal structures of IrI(CH3)(CF3)(CO)(PPh3)2, Ir(CF3)(C2F4)(CO)(PPh3)2 and Ir(CF3)(CF2)(CO)(PPh3)2. J. Organomet. Chem. 394, 615–642 (1990).
Hughes, R. P. et al. A simple route to difluorocarbene and perfluoroalkylidene complexes of iridium. J. Am. Chem. Soc. 127, 15020–15021 (2005).
Negishi, E. & de Meijere, A. Handbook of Organopalladium Chemistry for Organic Synthesis 2nd edn (Wiley, 2002).
Yang, Z.-Y., Wiemers, D. M. & Burton, D. J. Trifluoromethylcopper: a useful difluoromethylene transfer reagent: a novel double insertion of difluoromethylene into pentafluorophenylcopper. J. Am. Chem. Soc. 114, 4402–4403 (1992).
Zafrani, Y., Sod-Moriah, G. & Segall, Y. Diethyl bromodifluoromethylphosphonate: a highly efficient and environmentally benign difluorocarbene precursor. Tetrahedron 65, 5278–5283 (2009).
Cordaro, J. G. & Bergman, R. G. Dissociation of carbanions from acyl iridium compounds: an experimental and computational investigation. J. Am. Chem. Soc. 126, 16912–16929 (2004).
Fujiwara, Y. et al. A new reagent for direct difluoromethylation. J. Am. Chem. Soc. 134, 1494–1497 (2012).
Fier, P. S. & Hartwig, J. F. Copper-mediated difluoromethylation of aryl and vinyl iodides. J. Am. Chem. Soc. 134, 5524–5527 (2012).
Gu, Y., Leng, X. & Shen, Q. Cooperative dual palladium/silver catalyst for direct difluoromethylation of aryl bromides and iodides. Nat. Commun. 5, 5405 (2014).
Xu, L. & Vicic, D. A. Direct difluoromethylation of aryl halides via base metal catalysis at room temperature. J. Am. Chem. Soc. 138, 2536–2539 (2016).
Meanwell, N. A. Synopsis of some recent tactical application of bioisosteres in drug design. J. Med. Chem. 54, 2529–2591 (2011).
Erickson, J. A. & McLoughlin, J. I. Hydrogen bond donor properties of the difluoromethyl group. J. Org. Chem. 60, 1626–1631 (1995).
Vaclavik, J., Klimankova, I., Budinska, A. & Beier, P. Advances in the synthesis and application of tetrafluoroethylene- and 1,1,2,2-tetrafluoroethyl-containing compounds. Eur. J. Org. Chem. 2018, 3554–3593 (2018).
Ishiyama, T. et al. Mild iridium-catalyzed borylation of arenes. High turnover numbers, room temperature reactions and isolation of a potential intermediate. J. Am. Chem. Soc. 124, 390–391 (2002).
Jean, Y. Molecular Orbitals of Transition Metal Complexes (Oxford Univ. Press, 2005).
Li, L., Wang, F., Ni, C. & Hu, J. Synthesis of gem-difluorocyclopropa(e)nes and O-, S-, N- and P-difluoromethylated compounds with TMSCF2Br. Angew. Chem. Int. Ed. 52, 12390–12394 (2013).
Pu, M. I., Sanhueza, A., Senol, E. & Schoenebeck, F. Divergent reactivity of stannane and silane in the trifluoromethylation of PdII: cyclic transition state versus difluorocarbene release. Angew. Chem. Int. Ed. 57, 15081–15085 (2018).
Frisch, M. J. et al. Gaussian 09, revision D.01 (Gaussian, 2009).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B. 37, 785–789 (1988).
Becke, A. D. Density functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Becke, A. D. A new mixing of Hartree–Fock and local density-functional theories. J. Chem. Phys. 98, 1372–1377 (1993).
Hay, P. J. & Wadt, W. R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 82, 299–310 (1985).
Hehre, W. J., Ditchfield, R. & Pople, J. A. Self-consistent molecular orbital methods. XII. Further extensions of Gaussian-type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 56, 2257–2261 (1972).
Marenich, A. V., Cramer, C. J. & Truhlar, D. G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396 (2009).
Zhao, Y. & Truhlar, D. G. Density functionals with broad applicability in chemistry. Acc. Chem. Res. 41, 157–167 (2008).
Zhao, Y. & Truhlar, D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215–241 (2008).
Andrae, D., Haussermann, U., Dolg, M., Stoll, H. & Preuss, H. Energy-adjusted ab initio pseudo potentials for the second and third row transition elements. Theor. Chim. Acta 77, 123–141 (1990).
Dolg, M., Wedig, U., Stoll, H. & Preuss, H. Energy-adjusted ab initio pseudo potentials for the first row transition elements. J. Chem. Phys. 86, 866–872 (1987).
Legault, C. Y. CYLview, 1.0b (Université de Sherbrooke, 2009); http://www.cylview.org.
Acknowledgements
Financial support for this work was provided by the National Natural Science Foundation of China (21425208, 21672238, 21790362 and 21421002), the National Basic Research Program of China (973 Program) (No. 2015CB931900), the Strategic Priority Research Program of the Chinese Academy of Sciences (no. XDB20000000). We thank H.-L. Qin for MS analysis of 18O-labelled compound 7 and G.-Y. Li for 13C NMR analysis of the palladium complexes. K.N.H. acknowledges the National Science Foundation (NSF) for support (CHE-1764320). Computations were performed on the Hoffman2 cluster at UCLA and the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by the NSF (OCI-1053575).
Author information
Authors and Affiliations
Contributions
X.Z. and X.-P.F. conceived and designed the experiments. X.Z. directed the project. X.-P.F. performed the experiments and mechanism studies. X.-S.X. conducted the DFT calculations and contributed parts of the mechanism analysis. K.N.H. directed the DFT calculations. X.-Y.Z. conducted parts of the mechanistic studies. Y.-L.G. conducted MS analysis of the palladium complexes. X.L. analysed the X-ray crystal structure of the palladium difluorocarbene complex. X.-P.F., Y.-L.X. and X.Z. analysed the data. X.Z., X.-S.X., S.Z. and K.N.H. co-wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary experimental procedures, experimental data, optimization data, compound characterization data and computational methods.
A1-2.cif
Crystallographic information file for compound A1-2, CCDC 1916607.
B1-1.cif
Crystallographic information file for compound B1-1, CCDC 1902891.
B1-2.cif
Crystallographic information file for compound B1-2, CCDC 1916606.
C1-1a.cif
Crystallographic information file for compound C1-1a, CCDC 1902894.
C2.cif
Crystallographic information file for compound C2, CCDC 1902898.
cis-G1.cif
Crystallographic information file for compound cis-G1, CCDC 1902900.
E1.cif
Crystallographic information file for compound E1, CCDC 1902901.
Rights and permissions
About this article
Cite this article
Fu, XP., Xue, XS., Zhang, XY. et al. Controllable catalytic difluorocarbene transfer enables access to diversified fluoroalkylated arenes. Nat. Chem. 11, 948–956 (2019). https://doi.org/10.1038/s41557-019-0331-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-019-0331-9