当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Control of site selectivity in trifluoromethylation of alkenes bearing a pendant indolyl group: Synthesis of CF3-containing tetrahydrocarbazoles
Tetrahedron ( IF 2.1 ) Pub Date : 2019-01-23 , DOI: 10.1016/j.tet.2019.01.048 Ryo Murakami , Daisuke Sekine , Yuma Aoki , Shintaro Kawamura , Mikiko Sodeoka
中文翻译:

控制带有吲哚基侧基的烯烃在三氟甲基化中的位点选择性:含CF 3的四氢咔唑的合成
更新日期:2019-01-23
Tetrahedron ( IF 2.1 ) Pub Date : 2019-01-23 , DOI: 10.1016/j.tet.2019.01.048 Ryo Murakami , Daisuke Sekine , Yuma Aoki , Shintaro Kawamura , Mikiko Sodeoka
|
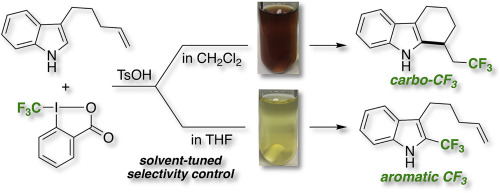
We present a tetrahydrocarbozole-forming carbo-trifluoromethylation of indoles bearing an alkenyl group at the C3 position. The reaction proceeded selectively with the combination of TsOH·H2O as a catalyst and CH2Cl2 as a solvent. The site-selectivity could be altered by changing the reaction solvent; the use of THF instead of CH2Cl2 increased the formation of aromatic trifluoromethylation products.
中文翻译:

控制带有吲哚基侧基的烯烃在三氟甲基化中的位点选择性:含CF 3的四氢咔唑的合成
我们提出了在C3位置带有烯基的吲哚的四氢咔唑形成碳三氟甲基化。通过将TsOH·H 2 O作为催化剂和CH 2 Cl 2作为溶剂,使反应选择性地进行。可以通过改变反应溶剂来改变位点选择性。使用THF代替CH 2 Cl 2可以增加芳族三氟甲基化产物的形成。





























 京公网安备 11010802027423号
京公网安备 11010802027423号