当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced Iodide Removal from Water by Nano-Silver Modified Anion Exchanger
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-12-10 , DOI: 10.1021/acs.iecr.8b04635 Jiao Li 1 , Manxiang Wang 2 , Guicheng Liu 3 , Liang Zhang 1 , Yali He 1 , Xing Xing 1 , Zhi Qian 1 , Jianzhong Zheng 1 , Congbin Xu 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-12-10 , DOI: 10.1021/acs.iecr.8b04635 Jiao Li 1 , Manxiang Wang 2 , Guicheng Liu 3 , Liang Zhang 1 , Yali He 1 , Xing Xing 1 , Zhi Qian 1 , Jianzhong Zheng 1 , Congbin Xu 1
Affiliation
|
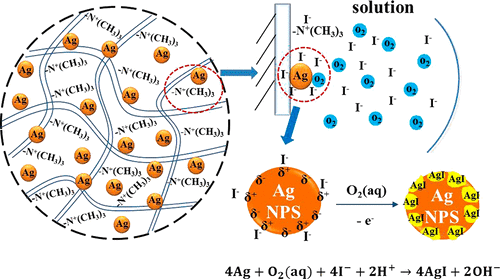
Water contamination by iodide has attracted much public attention in recent years due to its impact on public health. In this work, a novel sorbent Ag-D201 was synthesized by modifying a strong anion exchanger with nanosilver particles for selective iodide removal from water. Batch adsorption tests showed that, at neutral pH, Ag-D201 had a maximum iodide adsorption capacity of 312.5 mg/g. Solution pH had no obvious influence on iodide adsorption by this material in the pH range of 3–8, although further increase in solution pH would cause slight inhibition of iodide removal. Further experiments indicated that the material exhibited improved iodide removal selectivity in the presence of commonly encountered anions SO42–, NO3–, HCO3– and Cl– compared with its counterpart. At 500 mM chloride concentration (about 0.56 M Cl– in seawater), Ag-D201 still achieved more than half of its capacity as compared to control. A synergistic adsorption mechanism was proposed after analyzing the data from batch experiments and material characterizations using XPS and UV–vis. It was speculated that Ag-D201 synthesized in this study possessed two types of active sites for iodide adsorption, −N+(CH3)3 functional groups originally from the unmodified strong anion exchanger and the nanosilver particles impregnated in the resin matrix. The −N+(CH3)3 functional groups served to concentrate iodide from bulk solution to resin pores through electrostatic interaction, and nanosilver particles inside the resin pores attracted the neighboring iodide due to strong nucleophilic interaction between I– and Ag0. The appearance of strongly nucleophilic I– on silver surfaces helped to catalyze Ag oxidation by dissolved oxygen in solution, and eventually forming AgI precipitate, causing iodide removal from the system. This material would be potentially useful in treating iodide-contaminated water caused by accidental spills of high salinity water like that from deep geological formations during shale gas extraction.
中文翻译:

纳米银改性阴离子交换剂增强了水中的碘化物去除率
由于碘化物对公众健康的影响,近年来碘化物对水的污染引起了公众的广泛关注。在这项工作中,通过用纳米银颗粒修饰强阴离子交换剂以从水中选择性去除碘化物,合成了新型吸附剂Ag-D201。分批吸附测试表明,在中性pH下,Ag-D201的最大碘化物吸附容量为312.5 mg / g。在3-8的pH范围内,溶液的pH值对这种物质对碘化物的吸附没有明显影响,尽管溶液pH值的进一步增加会略微抑制碘化物的去除。进一步的实验表明,该材料中通常遇到的阴离子的存在表现出改善碘化物去除选择性SO 4 2-,NO 3 -,HCO 3 -和Cl –与之相对应。在500mM氯化钠的浓度(约0.56中号氯-在海水中),AG-D201仍与对照相比,实现了超过其容量的一半。在使用XPS和UV-vis分析批处理实验和材料表征中的数据后,提出了一种协同吸附机制。据推测,本研究中合成的Ag-D201具有两种类型的碘化物吸附活性位点,即-N +(CH 3)3官能团,其最初来自未改性的强阴离子交换剂,以及浸渍在树脂基质中的纳米银颗粒。-N +(CH 3)3官能团提供给树脂通过的孔隙的静电相互作用,以从本体溶液浓缩碘化物,和在树脂内部纳米银粒子细孔吸引相邻碘化物由于强亲核相互作用我之间-和Ag 0。在银表面上出现强亲核性I –有助于溶液中溶解的氧催化Ag氧化,并最终形成AgI沉淀,从而导致系统中碘化物的去除。这种材料在处理因高盐度水意外泄漏(如在页岩气提取过程中从深部地质层中意外溢出)而造成的碘化物污染水方面可能很有用。
更新日期:2018-12-11
中文翻译:

纳米银改性阴离子交换剂增强了水中的碘化物去除率
由于碘化物对公众健康的影响,近年来碘化物对水的污染引起了公众的广泛关注。在这项工作中,通过用纳米银颗粒修饰强阴离子交换剂以从水中选择性去除碘化物,合成了新型吸附剂Ag-D201。分批吸附测试表明,在中性pH下,Ag-D201的最大碘化物吸附容量为312.5 mg / g。在3-8的pH范围内,溶液的pH值对这种物质对碘化物的吸附没有明显影响,尽管溶液pH值的进一步增加会略微抑制碘化物的去除。进一步的实验表明,该材料中通常遇到的阴离子的存在表现出改善碘化物去除选择性SO 4 2-,NO 3 -,HCO 3 -和Cl –与之相对应。在500mM氯化钠的浓度(约0.56中号氯-在海水中),AG-D201仍与对照相比,实现了超过其容量的一半。在使用XPS和UV-vis分析批处理实验和材料表征中的数据后,提出了一种协同吸附机制。据推测,本研究中合成的Ag-D201具有两种类型的碘化物吸附活性位点,即-N +(CH 3)3官能团,其最初来自未改性的强阴离子交换剂,以及浸渍在树脂基质中的纳米银颗粒。-N +(CH 3)3官能团提供给树脂通过的孔隙的静电相互作用,以从本体溶液浓缩碘化物,和在树脂内部纳米银粒子细孔吸引相邻碘化物由于强亲核相互作用我之间-和Ag 0。在银表面上出现强亲核性I –有助于溶液中溶解的氧催化Ag氧化,并最终形成AgI沉淀,从而导致系统中碘化物的去除。这种材料在处理因高盐度水意外泄漏(如在页岩气提取过程中从深部地质层中意外溢出)而造成的碘化物污染水方面可能很有用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号