European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2018-09-26 , DOI: 10.1016/j.ejmech.2018.09.063 Adnan Ashraf , Syeda Abida Ejaz , Shafiq Ur Rahman , Waseeq Ahmad Siddiqui , Muhammad Nadeem Arshad , Joanna Lecka , Jean Sévigny , Mohie E. Moustafa Zayed , Abdullah M. Asiri , Jamshed Iqbal , Christian G. Hartinger , Muhammad Hanif
|
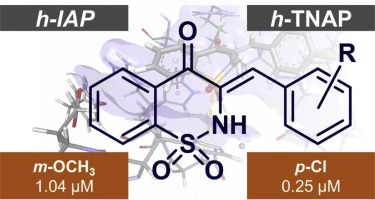
Chalcones and 1,2-benzothiazines are two important classes of bioactive compounds, each scaffold endowed with diverse pharmacological activities. Combining both of these pharmacophores in a single molecule was aimed to yield multi-modal agents. Herein, we report a series of hybrid compounds 3a–3o derived from chalcones and 1,2-benzothiazine cores. They were synthesized from commercially available sodium saccharin, and the resulting 1,2-benzothiazine-derived ketone was then condensed with aromatic aldehydes in an aldol condensation to obtain the respective chalcones. The compounds were characterized using different analytical techniques including FT-IR, NMR spectroscopy, mass spectrometry and X-ray crystallography. Some synthesized chalcones revealed potent and/or selective inhibitory properties towards alkaline phosphatase isozymes transiently expressed in COS-7 cells. A detailed structure-activity and selectivity study was carried out with regard to the effect of different substituents at ortho-, meta- and para-positions of the phenyl residue. Compound 3c was the most effective human intestinal alkaline phosphatase (h-IAP) inhibitor (IC50 value of 1.04 μM), while it was not active against human tissue non-specific alkaline phosphatase (h-TNAP) isozyme. In contrast, 3i was a selective inhibitor of h-TNAP with IC50 values of 0.25 ± 0.01 μM. The possible binding interactions of the most effective inhibitors of h-TNAP and h-IAP were obtained from molecular docking studies.
中文翻译:

查尔酮和1,2-苯并噻嗪药效基团的杂合化合物作为碱性磷酸酶同工酶的选择性抑制剂
查耳酮和1,2-苯并噻嗪是两类重要的生物活性化合物,每种支架都具有多种药理活性。将这两个药效基团组合在一个分子中的目的是产生多峰态药物。在此,我们报告了一系列杂化化合物3a – 3o衍生自查耳酮和1,2-苯并噻嗪核。它们由市售糖精钠合成,然后将得到的1,2-苯并噻嗪衍生的酮与芳香醛在醛醇缩合反应中缩合,得到相应的查耳酮。使用不同的分析技术(包括FT-IR,NMR光谱,质谱和X射线晶体学)对化合物进行表征。一些合成的查耳酮显示了对在COS-7细胞中瞬时表达的碱性磷酸酶同工酶的有效和/或选择性抑制特性。针对邻位,间位和对位不同取代基的影响,进行了详细的结构活性和选择性研究苯基残基的-位。化合物3c是最有效的人体肠道碱性磷酸酶(h -IAP)抑制剂(IC 50值为1.04μM),而对人体组织非特异性碱性磷酸酶(h -TNAP)同工酶没有活性。相反,3i是h -TNAP的选择性抑制剂,IC 50值为0.25±0.01μM。最有效的h -TNAP和h -IAP抑制剂可能的结合相互作用是从分子对接研究中获得的。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号