当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
18F-Labeling of Arenes and Heteroarenes for Applications in Positron Emission Tomography
Chemical Reviews ( IF 51.4 ) Pub Date : 2016-01-11 00:00:00 , DOI: 10.1021/acs.chemrev.5b00493 Sean Preshlock 1 , Matthew Tredwell 1 , Véronique Gouverneur 1
Chemical Reviews ( IF 51.4 ) Pub Date : 2016-01-11 00:00:00 , DOI: 10.1021/acs.chemrev.5b00493 Sean Preshlock 1 , Matthew Tredwell 1 , Véronique Gouverneur 1
Affiliation
|
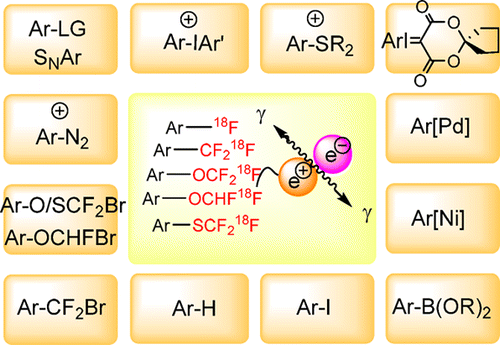
Diverse radiochemistry is an essential component of nuclear medicine; this includes imaging techniques such as positron emission tomography (PET). As such, PET can track diseases at an early stage of development, help patient care planning through personalized medicine and support drug discovery programs. Fluorine-18 is the most frequently used radioisotope in PET radiopharmaceuticals for both clinical and preclinical research. Its physical and nuclear characteristics (97% β+ decay, 109.8 min half-life, 635 keV positron energy) and high specific activity make it an attractive nuclide for labeling and molecular imaging. Arenes and heteroarenes are privileged candidates for 18F-incorporation as they are metabolically robust and therefore widely used by medicinal chemists and radiochemists alike. For many years, the range of (hetero)arenes amenable to 18F-fluorination was limited by the lack of chemically diverse precursors, and of radiochemical methods allowing 18F-incorporation in high selectivity and efficiency (radiochemical yield and purity, specific activity, and radio-scalability). The appearance of late-stage fluorination reactions catalyzed by transition metal or small organic molecules (organocatalysis) has encouraged much research on the use of these activation manifolds for 18F-fluorination. In this piece, we review all of the reactions known to date to install the 18F substituent and other key 18F-motifs (e.g., CF3, CHF2, OCF3, SCF3, OCHF2) of medicinal relevance onto (hetero)arenes. The field has changed significantly in the past five years, and the current trend suggests that the radiochemical space available for PET applications will expand rapidly in the near future.
中文翻译:

用于正电子发射断层扫描的18芳烃和杂芳烃的F标签
多种放射化学是核医学的重要组成部分。这包括成像技术,例如正电子发射断层扫描(PET)。因此,PET可以在开发的早期阶段跟踪疾病,通过个性化药物帮助患者护理计划并支持药物发现计划。氟18是用于临床和临床前研究的PET放射性药物中最常用的放射性同位素。它的物理和核特性(97%β +衰变,109.8分钟半衰期,635 keV正电子能量)和高比活度使其成为用于标记和分子成像的有吸引力的核素。芳烃和异芳烃是18岁的特权候选人F掺入具有代谢稳健性,因此被药物化学家和放射化学家广泛使用。许多年来,的(杂)芳烃的范围内适合于18 F-氟化因缺乏化学上不同的前体的限制,并且的放射化学方法使18中的高选择性和效率(放射化学产率和纯度,比活性F-掺入和无线电可扩展性)。过渡金属或有机小分子催化的后期氟化反应(有机催化)的出现鼓励了有关将这些活化歧管用于18 F氟化的大量研究。在本文中,我们回顾了迄今为止已知的所有安装18的反应。与(杂)芳烃具有医学相关性的F取代基和其他关键的18 F-基序(例如,CF 3,CHF 2,OCF 3,SCF 3,OCHF 2)。在过去的五年中,该领域发生了巨大变化,目前的趋势表明,可用于PET应用的放射化学领域将在不久的将来迅速扩展。
更新日期:2016-01-11
中文翻译:

用于正电子发射断层扫描的18芳烃和杂芳烃的F标签
多种放射化学是核医学的重要组成部分。这包括成像技术,例如正电子发射断层扫描(PET)。因此,PET可以在开发的早期阶段跟踪疾病,通过个性化药物帮助患者护理计划并支持药物发现计划。氟18是用于临床和临床前研究的PET放射性药物中最常用的放射性同位素。它的物理和核特性(97%β +衰变,109.8分钟半衰期,635 keV正电子能量)和高比活度使其成为用于标记和分子成像的有吸引力的核素。芳烃和异芳烃是18岁的特权候选人F掺入具有代谢稳健性,因此被药物化学家和放射化学家广泛使用。许多年来,的(杂)芳烃的范围内适合于18 F-氟化因缺乏化学上不同的前体的限制,并且的放射化学方法使18中的高选择性和效率(放射化学产率和纯度,比活性F-掺入和无线电可扩展性)。过渡金属或有机小分子催化的后期氟化反应(有机催化)的出现鼓励了有关将这些活化歧管用于18 F氟化的大量研究。在本文中,我们回顾了迄今为止已知的所有安装18的反应。与(杂)芳烃具有医学相关性的F取代基和其他关键的18 F-基序(例如,CF 3,CHF 2,OCF 3,SCF 3,OCHF 2)。在过去的五年中,该领域发生了巨大变化,目前的趋势表明,可用于PET应用的放射化学领域将在不久的将来迅速扩展。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号