当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Auxiliary-Directed Pd-Catalyzed γ-C(sp3)–H Bond Activation of α-Aminobutanoic Acid Derivatives
Organic Letters ( IF 4.9 ) Pub Date : 2015-12-04 00:00:00 , DOI: 10.1021/acs.orglett.5b03118 Kalyan Kumar Pasunooti 1 , Biplab Banerjee 1 , Terence Yap 1 , Yaojia Jiang 1 , Chuan-Fa Liu 1
Organic Letters ( IF 4.9 ) Pub Date : 2015-12-04 00:00:00 , DOI: 10.1021/acs.orglett.5b03118 Kalyan Kumar Pasunooti 1 , Biplab Banerjee 1 , Terence Yap 1 , Yaojia Jiang 1 , Chuan-Fa Liu 1
Affiliation
|
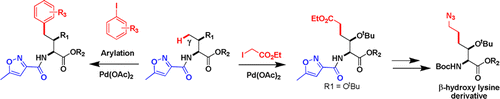
New bidentate auxiliaries derived from the isoxazole-3-carboxamide and oxazole-4-carboxamide moieties were used for Pd-catalyzed C(sp3)–H bond activation. The results show that, when placed on a primary amine compound, 5-methylisoxazole-3-carboxamide (MICA) directs Pd-catalyzed activation of inert γ-C(sp3)–H bonds for C–C bond formation. Selective and efficient arylation and alkylation of several α-aminobutanoic acid derivatives led to various γ-substituted non-natural amino acids. The MICA directing group can be conveniently removed and recovered under very mild conditions.
中文翻译:

辅助导向的Pd催化的α-氨基丁酸衍生物的γ-C(sp 3)–H键活化
衍生自异恶唑-3-甲酰胺和恶唑-4-甲酰胺基团的新双齿助剂用于Pd催化的C(sp 3)–H键活化。结果表明,当放置在伯胺化合物上时,5-甲基异恶唑-3-羧酰胺(MICA)指导Pd催化的惰性γ-C(sp 3)-H键的活化,从而形成C-C键。几种α-氨基丁酸衍生物的选择性和有效的芳基化和烷基化反应产生了各种γ-取代的非天然氨基酸。可以在非常温和的条件下方便地除去和回收MICA导向基团。
更新日期:2015-12-04
中文翻译:

辅助导向的Pd催化的α-氨基丁酸衍生物的γ-C(sp 3)–H键活化
衍生自异恶唑-3-甲酰胺和恶唑-4-甲酰胺基团的新双齿助剂用于Pd催化的C(sp 3)–H键活化。结果表明,当放置在伯胺化合物上时,5-甲基异恶唑-3-羧酰胺(MICA)指导Pd催化的惰性γ-C(sp 3)-H键的活化,从而形成C-C键。几种α-氨基丁酸衍生物的选择性和有效的芳基化和烷基化反应产生了各种γ-取代的非天然氨基酸。可以在非常温和的条件下方便地除去和回收MICA导向基团。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号