Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Viscoelasticity Response during Fibrillation of Amyloid β Peptides on a Quartz-Crystal-Microbalance Biosensor
Langmuir ( IF 3.7 ) Pub Date : 2018-04-26 00:00:00 , DOI: 10.1021/acs.langmuir.8b00639 Yen-Ting Lai 1 , Hirotsugu Ogi 2 , Kentaro Noi 2 , Fumihito Kato 3
Langmuir ( IF 3.7 ) Pub Date : 2018-04-26 00:00:00 , DOI: 10.1021/acs.langmuir.8b00639 Yen-Ting Lai 1 , Hirotsugu Ogi 2 , Kentaro Noi 2 , Fumihito Kato 3
Affiliation
|
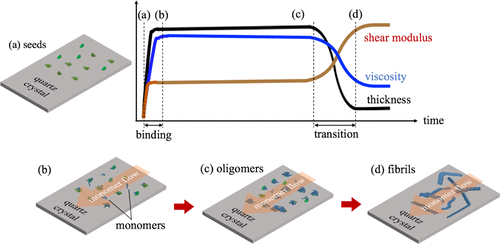
Unlike previous in vitro measurements where Amyloid β (Aβ) aggregation was studied in bulk solutions, we detect the structure change of the Aβ aggregate on the surface of a wireless quartz-crystal-microbalance biosensor, which resembles more closely the aggregation process on the cell membrane. Using a 58 MHz quartz crystal, we monitored changes in the viscoelastic properties of the aggregate formed on the quartz surface from monomers to oligomers and then to fibrils, involving up to the 7th overtone mode (406 MHz). With atomic-force microscopy observations, we found a significant stiffness increase as well as thinning of the protein layer during the structure change from oligomer to fibrils at 20 h, which indicates that the stiffness of the fibril is much higher. Viscoelasticity can provide a significant index of fibrillation and can be useful for evaluating inhibitory medicines in drug development.
中文翻译:

石英-晶体-微天平生物传感器上淀粉样β肽的原纤化过程中的粘弹性响应。
与以前的体外测量方法不同,该方法在大体积溶液中研究了淀粉样β(Aβ)聚集,我们检测无线石英晶体微天平生物传感器表面上Aβ聚集的结构变化,该变化更类似于细胞上的聚集过程膜。使用58 MHz的石英晶体,我们监测了在石英表面上形成的团聚体的粘弹性质的变化,从单体到低聚物,再到原纤维,涉及到第七个泛音模式(406 MHz)。借助原子力显微镜观察,我们发现在20 h从低聚物到原纤维的结构变化期间,蛋白质层的刚度显着增加,并且蛋白质层变薄,这表明原纤维的刚度要高得多。
更新日期:2018-04-26
中文翻译:

石英-晶体-微天平生物传感器上淀粉样β肽的原纤化过程中的粘弹性响应。
与以前的体外测量方法不同,该方法在大体积溶液中研究了淀粉样β(Aβ)聚集,我们检测无线石英晶体微天平生物传感器表面上Aβ聚集的结构变化,该变化更类似于细胞上的聚集过程膜。使用58 MHz的石英晶体,我们监测了在石英表面上形成的团聚体的粘弹性质的变化,从单体到低聚物,再到原纤维,涉及到第七个泛音模式(406 MHz)。借助原子力显微镜观察,我们发现在20 h从低聚物到原纤维的结构变化期间,蛋白质层的刚度显着增加,并且蛋白质层变薄,这表明原纤维的刚度要高得多。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号