当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Unified Approach for the Assembly of Atisine‐ and Hetidine‐type Diterpenoid Alkaloids: Total Syntheses of Azitine and the Proposed Structure of Navirine C
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-04-26 , DOI: 10.1002/anie.201803018 Jie Liu 1 , Dawei Ma 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-04-26 , DOI: 10.1002/anie.201803018 Jie Liu 1 , Dawei Ma 1
Affiliation
|
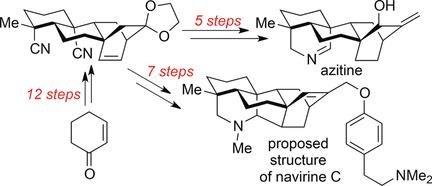
A tetracyclic dinitrile was synthesized in twelve steps from cyclohex‐2‐en‐1‐one by using a chelation‐triggered conjugate addition to a γ‐hydroxy‐substituted α,β‐unsaturated nitrile and an oxidative dearomatization/Diels–Alder cycloaddition cascade as the key steps. The first total synthesis of azitine (in 17 steps) was achieved through a simple reductive cyclization of this intermediate and subsequent transformations while the total synthesis of the proposed structure of navirine C (in 19 steps) was accomplished by a hydrogen‐atom‐transfer reaction of the tetracyclic dinitrile, Pd/C‐catalyzed reductive cyclization, and subsequent functional group manipulation.
中文翻译:

Atisine和Hetidine型二萜生物碱组装的统一方法:Azitine的总合成和Navirine C的拟议结构
通过从螯合触发的共轭物加成到γ-羟基取代的α,β-不饱和腈和氧化脱芳香化/ Diels-Alder环加成级联反应中,从螯合2-en-1一步十二步合成了四环二腈关键步骤。通过该中间体的简单还原环化和随后的转化,实现了首个叠氮基氮的全合成(共17个步骤),而通过氢原子转移反应完成了拟议的萘维林C结构的全合成(共19个步骤)。四环二腈的合成,Pd / C催化的还原环化以及随后的官能团操纵。
更新日期:2018-04-26
中文翻译:

Atisine和Hetidine型二萜生物碱组装的统一方法:Azitine的总合成和Navirine C的拟议结构
通过从螯合触发的共轭物加成到γ-羟基取代的α,β-不饱和腈和氧化脱芳香化/ Diels-Alder环加成级联反应中,从螯合2-en-1一步十二步合成了四环二腈关键步骤。通过该中间体的简单还原环化和随后的转化,实现了首个叠氮基氮的全合成(共17个步骤),而通过氢原子转移反应完成了拟议的萘维林C结构的全合成(共19个步骤)。四环二腈的合成,Pd / C催化的还原环化以及随后的官能团操纵。






























 京公网安备 11010802027423号
京公网安备 11010802027423号