Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Activity-based E3 ligase profiling uncovers an E3 ligase with esterification activity
Nature ( IF 50.5 ) Pub Date : 2018-04-01 , DOI: 10.1038/s41586-018-0026-1 Kuan-Chuan Pao 1 , Nicola T Wood 1 , Axel Knebel 1 , Karim Rafie 2 , Mathew Stanley 2 , Peter D Mabbitt 1 , Ramasubramanian Sundaramoorthy 2 , Kay Hofmann 3 , Daan M F van Aalten 2 , Satpal Virdee 1
Nature ( IF 50.5 ) Pub Date : 2018-04-01 , DOI: 10.1038/s41586-018-0026-1 Kuan-Chuan Pao 1 , Nicola T Wood 1 , Axel Knebel 1 , Karim Rafie 2 , Mathew Stanley 2 , Peter D Mabbitt 1 , Ramasubramanian Sundaramoorthy 2 , Kay Hofmann 3 , Daan M F van Aalten 2 , Satpal Virdee 1
Affiliation
|
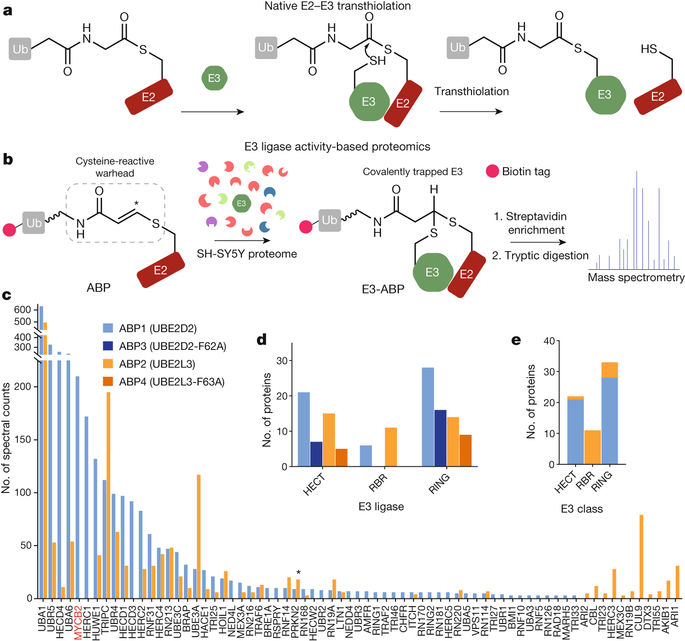
Ubiquitination is initiated by transfer of ubiquitin (Ub) from a ubiquitin-activating enzyme (E1) to a ubiquitin-conjugating enzyme (E2), producing a covalently linked intermediate (E2–Ub)1. Ubiquitin ligases (E3s) of the ‘really interesting new gene’ (RING) class recruit E2–Ub via their RING domain and then mediate direct transfer of ubiquitin to substrates2. By contrast, ‘homologous to E6-AP carboxy terminus’ (HECT) E3 ligases undergo a catalytic cysteine-dependent transthiolation reaction with E2–Ub, forming a covalent E3–Ub intermediate3,4. Additionally, RING-between-RING (RBR) E3 ligases have a canonical RING domain that is linked to an ancillary domain. This ancillary domain contains a catalytic cysteine that enables a hybrid RING–HECT mechanism5. Ubiquitination is typically considered a post-translational modification of lysine residues, as there are no known human E3 ligases with non-lysine activity. Here we perform activity-based protein profiling of HECT or RBR-like E3 ligases and identify the neuron-associated E3 ligase MYCBP2 (also known as PHR1) as the apparent single member of a class of RING-linked E3 ligase with esterification activity and intrinsic selectivity for threonine over serine. MYCBP2 contains two essential catalytic cysteine residues that relay ubiquitin to its substrate via thioester intermediates. Crystallographic characterization of this class of E3 ligase, which we designate RING-Cys-relay (RCR), provides insights into its mechanism and threonine selectivity. These findings implicate non-lysine ubiquitination in cellular regulation of higher eukaryotes and suggest that E3 enzymes have an unappreciated mechanistic diversity.Non-lysine ubiquitination activity of the E3 ubiquitin ligase MYCBP2 is identified by activity-based profiling; biochemical and structural analysis of MYCBP2 suggests the basis for its mechanism and specificity.
中文翻译:

基于活性的 E3 连接酶分析揭示了具有酯化活性的 E3 连接酶
泛素化是由泛素 (Ub) 从泛素激活酶 (E1) 转移到泛素结合酶 (E2),产生共价连接的中间体 (E2–Ub)1 启动的。“真正有趣的新基因”(RING) 类的泛素连接酶 (E3) 通过其 RING 结构域募集 E2–Ub,然后介导泛素向底物的直接转移2。相比之下,“与 E6-AP 羧基末端同源”(HECT) E3 连接酶与 E2-Ub 进行催化半胱氨酸依赖性转硫醇反应,形成共价 E3-Ub 中间体 3, 4。此外,RING-between-RING (RBR) E3 连接酶具有与辅助结构域相关联的规范 RING 结构域。该辅助结构域包含催化半胱氨酸,可实现混合 RING-HECT 机制 5。泛素化通常被认为是赖氨酸残基的翻译后修饰,因为没有已知的具有非赖氨酸活性的人类 E3 连接酶。在这里,我们对 HECT 或 RBR 样 E3 连接酶进行基于活性的蛋白质分析,并将神经元相关 E3 连接酶 MYCBP2(也称为 PHR1)鉴定为一类具有酯化活性和内在环连接酶的明显单一成员苏氨酸对丝氨酸的选择性。MYCBP2 包含两个必需的催化半胱氨酸残基,可通过硫酯中间体将泛素传递至其底物。此类 E3 连接酶(我们将其命名为 RING-Cys-relay (RCR))的晶体学表征提供了对其机制和苏氨酸选择性的深入了解。这些发现暗示了高等真核生物细胞调节中的非赖氨酸泛素化,并表明 E3 酶具有未被重视的机制多样性。E3 泛素连接酶 MYCBP2 的非赖氨酸泛素化活性通过基于活性的分析鉴定;MYCBP2 的生化和结构分析为其机制和特异性提供了基础。
更新日期:2018-04-01
中文翻译:

基于活性的 E3 连接酶分析揭示了具有酯化活性的 E3 连接酶
泛素化是由泛素 (Ub) 从泛素激活酶 (E1) 转移到泛素结合酶 (E2),产生共价连接的中间体 (E2–Ub)1 启动的。“真正有趣的新基因”(RING) 类的泛素连接酶 (E3) 通过其 RING 结构域募集 E2–Ub,然后介导泛素向底物的直接转移2。相比之下,“与 E6-AP 羧基末端同源”(HECT) E3 连接酶与 E2-Ub 进行催化半胱氨酸依赖性转硫醇反应,形成共价 E3-Ub 中间体 3, 4。此外,RING-between-RING (RBR) E3 连接酶具有与辅助结构域相关联的规范 RING 结构域。该辅助结构域包含催化半胱氨酸,可实现混合 RING-HECT 机制 5。泛素化通常被认为是赖氨酸残基的翻译后修饰,因为没有已知的具有非赖氨酸活性的人类 E3 连接酶。在这里,我们对 HECT 或 RBR 样 E3 连接酶进行基于活性的蛋白质分析,并将神经元相关 E3 连接酶 MYCBP2(也称为 PHR1)鉴定为一类具有酯化活性和内在环连接酶的明显单一成员苏氨酸对丝氨酸的选择性。MYCBP2 包含两个必需的催化半胱氨酸残基,可通过硫酯中间体将泛素传递至其底物。此类 E3 连接酶(我们将其命名为 RING-Cys-relay (RCR))的晶体学表征提供了对其机制和苏氨酸选择性的深入了解。这些发现暗示了高等真核生物细胞调节中的非赖氨酸泛素化,并表明 E3 酶具有未被重视的机制多样性。E3 泛素连接酶 MYCBP2 的非赖氨酸泛素化活性通过基于活性的分析鉴定;MYCBP2 的生化和结构分析为其机制和特异性提供了基础。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号