Cell Reports ( IF 7.5 ) Pub Date : 2019-11-19 , DOI: 10.1016/j.celrep.2019.10.055 You Wu 1 , Wenqiang Liu 2 , Jiayu Chen 2 , Shuaitong Liu 1 , Mingzhu Wang 1 , Lei Yang 1 , Chuan Chen 1 , Meijie Qi 3 , Yiwen Xu 4 , Zhibin Qiao 1 , Rushuang Yan 1 , Xiaochen Kou 1 , Yanhong Zhao 1 , Bin Shen 3 , Jiqing Yin 1 , Hong Wang 1 , Yawei Gao 1 , Shaorong Gao 5
|
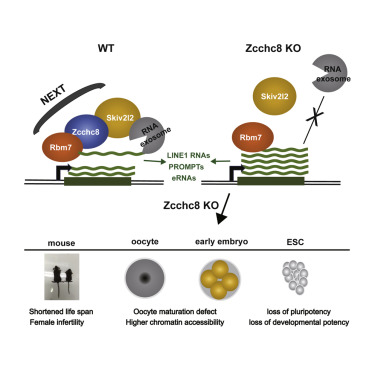
The nuclear exosome targeting (NEXT) complex is responsible for specific nuclear RNA degradation in mammalian cells. However, its function in development remains unknown. Here, we find that the depletion of a central factor of the NEXT complex, Zcchc8, in mouse results in developmental defects, a shortened lifespan, and infertility. We find that Zcchc8-deficient embryonic stem cells (ESCs) exhibit proliferation abnormalities and reduced developmental potencies. Importantly, the transcripts of retrotransposon element LINE1 are found to be targeted by Zcchc8 and degraded by a Zcchc8-mediated mechanism. We further find that sustained expression of higher levels of LINE1 RNA is detected in maternal Zcchc8-depleted oocytes and embryos. Zcchc8-depleted oocytes show higher chromatin accessibility and developmental defects in both meiotic maturation and embryogenesis after fertilization. Collectively, our study defines Zcchc8-mediated RNA degradation as an important post-transcription regulation of LINE1 transcripts in early embryos and ESCs, which play vital roles in the pluripotency and early development.
中文翻译:

核外泌体靶向复杂核心因子Zcchc8调节早期胚胎和胚胎干细胞中LINE1 RNA的降解。
核外泌体靶向(NEXT)复合体负责哺乳动物细胞中特定的核RNA降解。但是,其在开发中的功能仍然未知。在这里,我们发现小鼠中NEXT复合物Zcchc8的中心因子的消耗导致发育缺陷,寿命缩短和不育。我们发现Zcchc8缺陷的胚胎干细胞(ESC)表现出增殖异常和减少的发展潜力。重要的是,发现反转录转座子元件LINE1的转录物被Zcchc8靶向,并被Zcchc8介导的机制降解。我们进一步发现,在母体Zcchc8耗竭的卵母细胞和胚胎中检测到了LINE1 RNA高水平的持续表达。Zcchc8耗尽的卵母细胞在受精后的减数分裂成熟和胚胎发生中显示出更高的染色质可及性和发育缺陷。总的来说,我们的研究将Zcchc8介导的RNA降解定义为早期胚胎和ESC中LINE1转录物的重要转录后调控,这在多能性和早期发育中起着至关重要的作用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号