当前位置:
X-MOL 学术
›
Small Methods
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Situ Ag Nanoparticles Reinforced Pseudo‐Zn–Air Reaction Boosting Ag2V4O11 as High‐Performance Cathode Material for Aqueous Zinc‐Ion Batteries
Small Methods ( IF 10.7 ) Pub Date : 2019-11-11 , DOI: 10.1002/smtd.201900637 Qian Li 1 , Yuyi Liu 1 , Kaixuan Ma 1 , Gongzheng Yang 1 , Chengxin Wang 1, 2
Small Methods ( IF 10.7 ) Pub Date : 2019-11-11 , DOI: 10.1002/smtd.201900637 Qian Li 1 , Yuyi Liu 1 , Kaixuan Ma 1 , Gongzheng Yang 1 , Chengxin Wang 1, 2
Affiliation
|
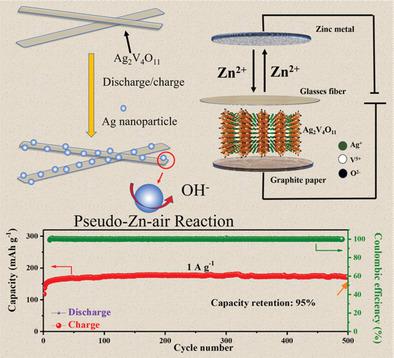
Aqueous zinc‐ion batteries (AZIBs), which are low‐cost and environmentally friendly, have been regarded feasible for large‐scale energy storage. But the widespread application of AZIBs is hindered by lack of suitable cathode materials with high capacity and long cycle life. The zinc‐storage mechanisms, especially the formation of basic zinc salt (BZS), are still unclear. Here, Ag2V4O11 is developed as a cathode material for AZIBs, which delivers a specific capacity of 213 mA h g−1 and excellent cycling performance (93% capacity retention after 6000 cycles). The reversible formation/decomposition of BZS and reduction/oxidation of metallic Ag are ascertained during the insertion/extraction of Zn(H2O)62+. Remarkably, the phase composition of BZS in Zn(CF3SO3)2‐based electrolyte is identified first. The role of in situ formed Ag nanoparticles is simulated by employing the commercial Ag nanoparticles as an additive into the V2O5‐based electrodes. The introduction of Ag significantly improves the specific capacity (at least 50% improvement) and accordingly it is proposed that the pseudo‐Zn–air reaction (oxygen reduction reaction‐like redox reaction happens on material surface in a closed system) promotes the electrochemical performance of Ag2V4O11. This work reveals the BZS rather than unknown new phases on the electrode surface and puts forward a possible way in raising electrochemical properties by utilizing the pseudo‐Zn–air reaction.
中文翻译:

原位银纳米颗粒增强的伪锌-空气反应增强了Ag2V4O11作为水性锌离子电池的高性能阴极材料
低成本且环保的锌离子水电池(AZIBs)被认为可用于大规模储能。但是由于缺乏合适的阴极材料,其容量大,循环寿命长,阻碍了AZIBs的广泛应用。锌的储存机制,特别是碱性锌盐(BZS)的形成,仍不清楚。在这里,Ag 2 V 4 O 11被开发为AZIBs的阴极材料,其比容量为213 mA hg -1,具有出色的循环性能(6000次循环后容量保持率达93%)。在Zn(H 2 O)的插入/萃取过程中,确定了BZS的可逆形成/分解和金属Ag的还原/氧化。6 2+。值得注意的是,首先确定了Zn(CF 3 SO 3)2基电解质中BZS的相组成。通过使用商业化的Ag纳米粒子作为添加剂添加到基于V 2 O 5的电极中,可以模拟原位形成的Ag纳米粒子的作用。Ag的引入显着提高了比容量(至少提高了50%),因此提出拟锌-空气反应(密闭系统中的材料表面发生氧还原反应样氧化还原反应)可提高电化学性能。的Ag 2 V 4 O 11。这项工作揭示了电极表面上的BZS而不是未知的新相,并提出了一种利用拟锌-空气反应提高电化学性能的可能方法。
更新日期:2019-11-11
中文翻译:

原位银纳米颗粒增强的伪锌-空气反应增强了Ag2V4O11作为水性锌离子电池的高性能阴极材料
低成本且环保的锌离子水电池(AZIBs)被认为可用于大规模储能。但是由于缺乏合适的阴极材料,其容量大,循环寿命长,阻碍了AZIBs的广泛应用。锌的储存机制,特别是碱性锌盐(BZS)的形成,仍不清楚。在这里,Ag 2 V 4 O 11被开发为AZIBs的阴极材料,其比容量为213 mA hg -1,具有出色的循环性能(6000次循环后容量保持率达93%)。在Zn(H 2 O)的插入/萃取过程中,确定了BZS的可逆形成/分解和金属Ag的还原/氧化。6 2+。值得注意的是,首先确定了Zn(CF 3 SO 3)2基电解质中BZS的相组成。通过使用商业化的Ag纳米粒子作为添加剂添加到基于V 2 O 5的电极中,可以模拟原位形成的Ag纳米粒子的作用。Ag的引入显着提高了比容量(至少提高了50%),因此提出拟锌-空气反应(密闭系统中的材料表面发生氧还原反应样氧化还原反应)可提高电化学性能。的Ag 2 V 4 O 11。这项工作揭示了电极表面上的BZS而不是未知的新相,并提出了一种利用拟锌-空气反应提高电化学性能的可能方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号