Cell Reports ( IF 7.5 ) Pub Date : 2019-10-30 , DOI: 10.1016/j.celrep.2019.09.054 Vanesa Álvarez 1 , Camilla Frattini 2 , María P Sacristán 3 , Alfonso Gallego-Sánchez 1 , Rodrigo Bermejo 2 , Avelino Bueno 3
|
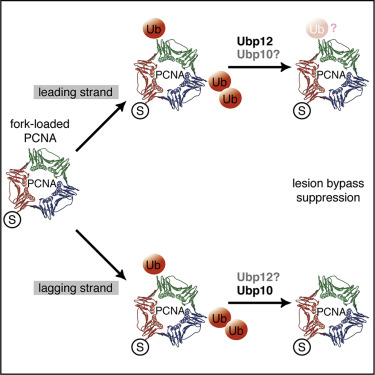
DNA damage tolerance plays a key role in protecting cell viability through translesion synthesis and template switching-mediated bypass of genotoxic polymerase-blocking base lesions. Both tolerance pathways critically rely on ubiquitylation of the proliferating-cell nuclear antigen (PCNA) on lysine 164 and have been proposed to operate uncoupled from replication. We report that Ubp10 and Ubp12 ubiquitin proteases differentially cooperate in PCNA deubiquitylation, owing to distinct activities on PCNA-linked ubiquitin chains. Ubp10 and Ubp12 associate with replication forks in a fashion determined by Ubp10 dependency on lagging-strand PCNA residence, and they downregulate translesion polymerase recruitment and template switch events engaging nascent strands. These findings reveal PCNAK164 deubiquitylation as a key mechanism for the modulation of lesion bypass during replication, which might set a framework for establishing strand-differential pathway choices. We propose that damage tolerance is tempered at replication forks to limit the extension of bypass events and sustain chromosome replication rates.
中文翻译:

PCNA去泛素化酶控制复制叉处的DNA损伤旁路。
DNA损伤耐受性通过转基因合成和模板切换介导的遗传毒性聚合酶阻滞性基础病变的旁路保护,在保护细胞活力中起着关键作用。两种耐受途径都关键地依赖赖氨酸164上增殖细胞核抗原(PCNA)的泛素化作用,并且已提出它们可以与复制脱钩地操作。我们报告说,Ubp10和Ubp12泛素蛋白酶在PCNA去泛素化中有差异地合作,这归因于PCNA连接的泛素链上的不同活性。Ubp10和Ubp12与复制叉相关联,其方式取决于Ubp10对滞后链PCNA驻留的依赖性,并且它们下调跨病变聚合酶募集和参与新生链的模板转换事件。这些发现揭示了PCNA K164去泛素化是复制过程中调节病变旁路的关键机制,这可能为建立链差异途径选择奠定了框架。我们建议在复制叉处减轻损伤的耐受性,以限制旁路事件的扩展并维持染色体复制率。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号