当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxindole‐chromones C3 Synthons Directed Stereocontrolled Construction of Five Contiguous Stereocenters on Spiro[tetrahydrocyclopenta[b]chromanone‐oxindole]s
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-10-22 , DOI: 10.1002/adsc.201901091 Sheng‐Wen Xu 1 , Xiong‐Wei Liu 2 , Xiong Zuo 1 , Gen Zhou 1 , Yi Gong 1, 2 , Xiong‐Li Liu 1 , Ying Zhou 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-10-22 , DOI: 10.1002/adsc.201901091 Sheng‐Wen Xu 1 , Xiong‐Wei Liu 2 , Xiong Zuo 1 , Gen Zhou 1 , Yi Gong 1, 2 , Xiong‐Li Liu 1 , Ying Zhou 2
Affiliation
|
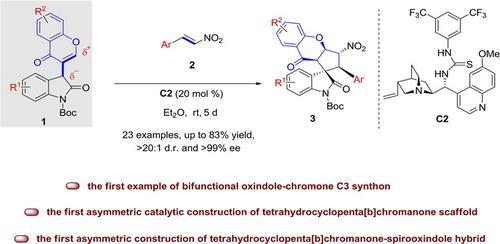
The first example of bifunctional oxindole‐chromone C3 synthon directed organocatalytic cascade cycloaddition reaction is developed, serving as a fruitful strategy for the facile access of optically active tetrahydrocyclopenta[b]chromanone bearing one spirooxindole with five contiguous stereocenters. All the products are smoothly obtained with up to 83% yield, >20:1 d.r. and >99% ee. This is also the first asymmetric catalytic construction of tetrahydrocyclopenta[b] chromanone and tetrahydrocyclopenta[b]chromanone‐spirooxindole scaffold, thus to facilitate the search for new bioactive entities.
中文翻译:

氧吲哚-色酮C3合成子在螺[tetrahydrocyclopenta [b] chromanone-oxindole] s上定向立体控制五个连续立体中心的构建
开发了第一个双功能羟吲哚-色酮C3合成子指导的有机催化级联环加成反应的实例,为轻松获得带有一个带有五个连续立体中心的螺氧杂吲哚的旋光四氢环戊[b]苯并二氢吡喃酮提供了富有成效的策略。顺利获得所有产品,收率高达83%,dr> 20:1和ee> 99%。这也是四氢环戊并[b]苯并二氢吡喃并四氢环戊并[b]苯并二氢吡喃-螺并恶唑骨架的第一个不对称催化结构,从而有利于寻找新的生物活性实体。
更新日期:2019-10-23
中文翻译:

氧吲哚-色酮C3合成子在螺[tetrahydrocyclopenta [b] chromanone-oxindole] s上定向立体控制五个连续立体中心的构建
开发了第一个双功能羟吲哚-色酮C3合成子指导的有机催化级联环加成反应的实例,为轻松获得带有一个带有五个连续立体中心的螺氧杂吲哚的旋光四氢环戊[b]苯并二氢吡喃酮提供了富有成效的策略。顺利获得所有产品,收率高达83%,dr> 20:1和ee> 99%。这也是四氢环戊并[b]苯并二氢吡喃并四氢环戊并[b]苯并二氢吡喃-螺并恶唑骨架的第一个不对称催化结构,从而有利于寻找新的生物活性实体。





























 京公网安备 11010802027423号
京公网安备 11010802027423号