Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2019-10-08 , DOI: 10.1016/j.bmc.2019.115146 Louisa Temme 1 , Frederik Börgel 2 , Dirk Schepmann 2 , Dina Robaa 3 , Wolfgang Sippl 3 , Constantin Daniliuc 4 , Bernhard Wünsch 1
|
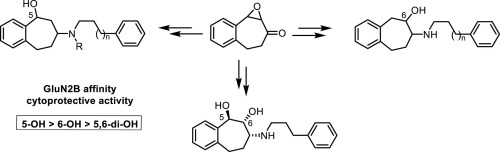
In this study, the impact of one or two hydroxy moieties at the benzo[7]annulene scaffold on the GluN2B affinity and cytoprotective activity was analyzed. The key intermediate for the synthesis of OH-substituted benzo[7]annulenamines 11-13 and 17 was the epoxyketone 8. Reductive epoxide opening of 8 resulted with high regioselectivity in the 5-hydroxyketone 9 (Pd(OAc)2, HCO2H, phosphane ligand) or the 6-hydroxyketone 10 (H2, Pd/C), whereas hydrolysis in aqueous dioxane led to the dihydroxyketone 14. Reductive amination of these ketones with primary amines and NaBH(OAc)3 afforded the benzo[7]annulenamines 11-13 and 17. In receptor binding studies 5-OH derivatives 11 and 12 showed higher GluN2B affinity than 6-OH derivatives 13, which in turn were more active than 5,6-di-OH derivative 17a. The same order was found for the cytoprotective activity of the ligands. The tertiary amine 12a with one OH moiety in 5-position represents the most promising GluN2B negative allosteric modulator with a binding affinity of Ki = 49 nM and a cytoprotective activity of IC50 = 580 nM. In the binding pocket 12a shows a crucial H-bond between the benzylic OH moiety and the backbone carbonyl O-atom of Ser132 (GluN1b). It was concluded that a 5-OH moiety is essential for the inhibition of the NMDA receptor associated ion channel, whereas a OH moiety in 6-position is detrimental for binding and inhibition. An OH or CH2OH moiety at 2-position results in binding at the ifenprodil binding site, but very weak ion channel inhibition.
中文翻译:

羟基部分对GluN2B配体的苯并[7]环戊烯环系统的影响:设计,合成和生物学评估。
在这项研究中,分析了苯并[7]环戊烯骨架上一个或两个羟基对GluN2B亲和力和细胞保护活性的影响。为OH取代的苯并[7]的合成中间体的关键annulenamines 11 - 13和17是环氧酮8。在5-羟基酮9(Pd(OAc)2,HCO 2 H,膦配体)或6-羟基酮10(H 2,Pd / C)中,还原环氧化物8的开环具有较高的区域选择性,而在二恶烷水溶液中的水解则导致二羟基酮14。这些酮与伯胺和NaBH(OAc)的还原性胺化3得到苯并[7] annulenamines 11 - 13和17。在受体结合研究中,5-OH衍生物11和12显示出比6-OH衍生物13高的GluN2B亲和力,而6-OH衍生物13则比5,6-di-OH衍生物17a更具活性。对于配体的细胞保护活性,发现了相同的顺序。在5位上带有一个OH部分的叔胺12a代表最有希望的GluN2B负变构调节剂,其结合亲和力为K i = 49 nM,细胞保护活性为IC 50 = 580 nM。在结合口袋12a中,在苄基OH部分和Ser132(GluN1b)的骨架羰基O原子之间显示了关键的H键。结论是5-OH部分对于抑制NMDA受体相关的离子通道是必不可少的,而6-位的OH部分对于结合和抑制是有害的。在2位的OH或CH 2 OH部分导致在艾芬地尔的结合位点结合,但对离子通道的抑制作用非常弱。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号