当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrically Conductive Shell-Protective Layer Capping on the Silicon Surface as the Anode Material for High-Performance Lithium-Ion Batteries
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-10-16 00:00:00 , DOI: 10.1021/acsami.9b13941 Ruiqi Na 1 , Krysten Minnici , Guoyan Zhang , Nan Lu 1 , Miguel A. González , Guibin Wang 1 , Elsa Reichmanis
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-10-16 00:00:00 , DOI: 10.1021/acsami.9b13941 Ruiqi Na 1 , Krysten Minnici , Guoyan Zhang , Nan Lu 1 , Miguel A. González , Guibin Wang 1 , Elsa Reichmanis
Affiliation
|
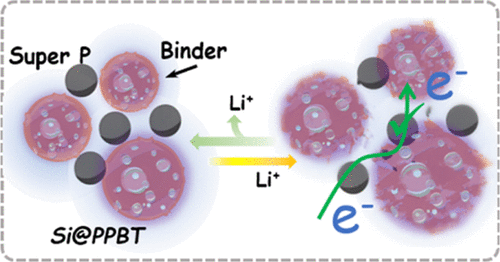
Rational design and construction of effective silicon (Si) electrode structures to relieve large volumetric changes that occur during the charge/discharge process remain significant challenges for the development of robust lithium-ion batteries (LIBs). Herein, we propose an electrically conductive poly[3-(potassium-4-butanoate)thiophene] (PPBT) capping layer on the Si surface (Si@PPBT) to serve as the active material and be used in conjunction with a common polymer binder as an approach to tackle issues emanating from volumetric changes. The PPBT protective shell layer provides the system with tolerance toward variations in active material volume during cycling, improves the dispersion of Si nanoparticles in the binder, enhances the electrolyte uptake rate, and provides a strong adhesion force between the Si/carbon additives/current collector, thereby helping to maintain electrode integrity during the charge/discharge process. The π-conjugated polythiophene backbone structure also allows the Si core to maintain electrical contact with carbon additives and/or polymer binder, enabling the formation of effective electrical transport bridges and stabilizing solid electrolyte interphase layer growth. The integrated Si@PPBT/carboxymethyl cellulose (CMC) anode exhibited high initial Coulombic efficiency (84.9%), enhanced rate capability performance, and long cycling stability with a reversible capacity of 1793 mA h g–1 after 200 cycles, 3.4 times higher than that of pristine Si anodes with the same CMC binder (528 mA h g–1). The results suggest that the Si@PPBT design presents a promising approach to promote the practical use of Si anodes in LIBs, which could be extended to other anode materials exhibiting large volume changes during lithiation/delithiation.
中文翻译:

覆盖在硅表面上的导电壳保护层作为高性能锂离子电池的阳极材料
有效的硅(Si)电极结构的合理设计和构造,以缓解充放电过程中发生的大体积变化,对于开发坚固的锂离子电池(LIB)仍然是重大挑战。本文中,我们建议在Si表面(Si @ PPBT)上使用导电的聚[3-(4-丁酸钾)噻吩](PPBT)覆盖层作为活性材料,并与常见的聚合物粘合剂一起使用作为解决体积变化引起的问题的一种方法。PPBT保护壳层为系统提供了对循环过程中活性物质体积变化的耐受性,改善了Si纳米颗粒在粘合剂中的分散性,提高了电解质的吸收速率,并在Si /碳添加剂/集流体之间提供了强大的粘附力,从而有助于在充电/放电过程中保持电极完整性。π-共轭聚噻吩主链结构还允许Si核与碳添加剂和/或聚合物粘合剂保持电接触,从而能够形成有效的电传输桥并稳定固体电解质相间层的生长。集成的Si @ PPBT /羧甲基纤维素(CMC)阳极具有很高的初始库仑效率(84.9%),增强的倍率性能和长循环稳定性,可逆容量为1793 mA hg 能够形成有效的电传输桥,并稳定固体电解质相间层的生长。集成的Si @ PPBT /羧甲基纤维素(CMC)阳极具有很高的初始库仑效率(84.9%),增强的倍率性能和长循环稳定性,可逆容量为1793 mA hg 能够形成有效的电传输桥,并稳定固体电解质相间层的生长。集成的Si @ PPBT /羧甲基纤维素(CMC)阳极具有很高的初始库仑效率(84.9%),增强的倍率性能和长循环稳定性,可逆容量为1793 mA hg200个循环后为–1,比具有相同CMC粘合剂(528 mA hg –1)的原始Si阳极高3.4倍。结果表明,Si @ PPBT设计提出了一种有前途的方法,以促进LIB中Si阳极的实际使用,该方法可扩展到在锂化/脱锂过程中表现出大体积变化的其他阳极材料。
更新日期:2019-10-17
中文翻译:

覆盖在硅表面上的导电壳保护层作为高性能锂离子电池的阳极材料
有效的硅(Si)电极结构的合理设计和构造,以缓解充放电过程中发生的大体积变化,对于开发坚固的锂离子电池(LIB)仍然是重大挑战。本文中,我们建议在Si表面(Si @ PPBT)上使用导电的聚[3-(4-丁酸钾)噻吩](PPBT)覆盖层作为活性材料,并与常见的聚合物粘合剂一起使用作为解决体积变化引起的问题的一种方法。PPBT保护壳层为系统提供了对循环过程中活性物质体积变化的耐受性,改善了Si纳米颗粒在粘合剂中的分散性,提高了电解质的吸收速率,并在Si /碳添加剂/集流体之间提供了强大的粘附力,从而有助于在充电/放电过程中保持电极完整性。π-共轭聚噻吩主链结构还允许Si核与碳添加剂和/或聚合物粘合剂保持电接触,从而能够形成有效的电传输桥并稳定固体电解质相间层的生长。集成的Si @ PPBT /羧甲基纤维素(CMC)阳极具有很高的初始库仑效率(84.9%),增强的倍率性能和长循环稳定性,可逆容量为1793 mA hg 能够形成有效的电传输桥,并稳定固体电解质相间层的生长。集成的Si @ PPBT /羧甲基纤维素(CMC)阳极具有很高的初始库仑效率(84.9%),增强的倍率性能和长循环稳定性,可逆容量为1793 mA hg 能够形成有效的电传输桥,并稳定固体电解质相间层的生长。集成的Si @ PPBT /羧甲基纤维素(CMC)阳极具有很高的初始库仑效率(84.9%),增强的倍率性能和长循环稳定性,可逆容量为1793 mA hg200个循环后为–1,比具有相同CMC粘合剂(528 mA hg –1)的原始Si阳极高3.4倍。结果表明,Si @ PPBT设计提出了一种有前途的方法,以促进LIB中Si阳极的实际使用,该方法可扩展到在锂化/脱锂过程中表现出大体积变化的其他阳极材料。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号