当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
1-Methyl-1,4-cyclohexadiene as a Traceless Reducing Agent for the Synthesis of Catechols and Hydroquinones.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-10-02 , DOI: 10.1021/acs.joc.9b01898 Andrea Baschieri 1 , Riccardo Amorati 1 , Luca Valgimigli 1 , Letizia Sambri 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-10-02 , DOI: 10.1021/acs.joc.9b01898 Andrea Baschieri 1 , Riccardo Amorati 1 , Luca Valgimigli 1 , Letizia Sambri 2
Affiliation
|
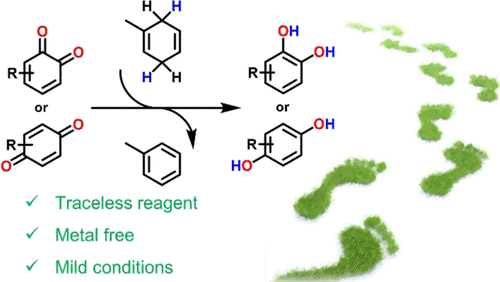
Pro-aromatic and volatile 1-methyl-1,4-cyclohexadiene (MeCHD) was used for the first time as a valid H-atom source in an innovative method to reduce ortho or para quinones to obtain the corresponding catechols and hydroquinones in good to excellent yields. Notably, the excess of MeCHD and the toluene formed as the oxidation product can be easily removed by evaporation. In some cases, trifluoroacetic acid as a catalyst was added to obtain the desired products. The reaction proceeds in air and under mild conditions, without metal catalysts and sulfur derivatives, resulting in an excellent and competitive method to reduce quinones. The mechanism is attributed to a radical reaction triggered by a hydrogen atom transfer from MeCHD to quinones, or, in the presence of trifluoroacetic acid, to a hydride transfer process.
中文翻译:

1-甲基-1,4-环己二烯作为无痕量还原剂,用于合成邻苯二酚和对苯二酚。
芳香族和挥发性的1-甲基-1,4-环己二烯(MeCHD)首次作为一种有效的H原子来源用于一种新颖的还原邻或对醌的方法,从而获得相应的邻苯二酚和对苯二酚,优异的产量。值得注意的是,通过蒸发可以容易地除去过量的MeCHD和作为氧化产物形成的甲苯。在某些情况下,添加三氟乙酸作为催化剂以获得所需的产物。该反应在空气中和温和条件下进行,没有金属催化剂和硫衍生物,因此是一种极好的竞争性方法来还原醌。该机理归因于自由基反应,该自由基反应是由氢从MeCHD转移至醌引发的,或在三氟乙酸存在下的氢化物转移过程。
更新日期:2019-10-02
中文翻译:

1-甲基-1,4-环己二烯作为无痕量还原剂,用于合成邻苯二酚和对苯二酚。
芳香族和挥发性的1-甲基-1,4-环己二烯(MeCHD)首次作为一种有效的H原子来源用于一种新颖的还原邻或对醌的方法,从而获得相应的邻苯二酚和对苯二酚,优异的产量。值得注意的是,通过蒸发可以容易地除去过量的MeCHD和作为氧化产物形成的甲苯。在某些情况下,添加三氟乙酸作为催化剂以获得所需的产物。该反应在空气中和温和条件下进行,没有金属催化剂和硫衍生物,因此是一种极好的竞争性方法来还原醌。该机理归因于自由基反应,该自由基反应是由氢从MeCHD转移至醌引发的,或在三氟乙酸存在下的氢化物转移过程。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号