Tetrahedron Letters ( IF 1.5 ) Pub Date : 2019-09-17 , DOI: 10.1016/j.tetlet.2019.151165 Yuanyuan Li , Bingxia Sun , Jiadai Zhai , Lin Fu , Shuxin Zhang , Jing Zhang , Hongliang Liu , Wenhai Xie , Hongkuan Deng , Zhiwei Chen , Feng Sang
|
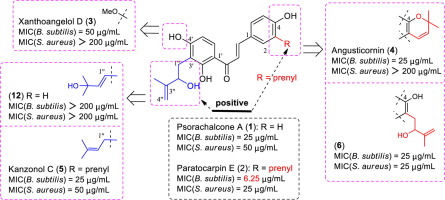
Four natural chalcones bearing hydroxyisoprenyl or prenyl groups, named Paratocarpin E (2), Xanthoangelol D (3), Angusticornin A (4) and Kanzonol C (5), were prepared by employing the Claisen-Schmidt condensation as the key step. In an attempt to investigate the effect of the hydroxyisoprenyl group on biological activity, two of their derivatives were also prepared for antibacterial activity research. The synthesized compounds were investigated for their expected antibacterial activities against Gram positive bacteria (Bacillus subtilis, Staphylococcus aureus) as well as Gram negative bacteria (Escherichia coli, Pseudomonas aeruginosa). Paratocarpin E (2) was found to be the most potent against two Gram positive bacteria while the majority of the remaining compounds showed promising activity as well. However, all of the compounds were inactive against both Gram-negative bacteria.
中文翻译:

四种天然查耳酮及其衍生物的合成及抑菌活性
通过使用克莱森-施密特缩合作为关键步骤,制备了四个带有羟基异戊二烯基或异戊二烯基的天然查耳酮,分别命名为对果皮素E(2),黄嘌呤酚D(3),安格斯汀A(4)和坎佐诺尔C(5)。为了研究羟基异戊二烯基对生物活性的影响,还制备了它们的两种衍生物用于抗菌活性研究。研究了合成化合物对革兰氏阳性细菌(枯草芽孢杆菌,金黄色葡萄球菌)和革兰氏阴性细菌(大肠杆菌,铜绿假单胞菌)的预期抗菌活性。)。已发现对果肉中的E(2)对两种革兰氏阳性细菌最有效,而其余大多数化合物也显示出有希望的活性。但是,所有化合物均对两种革兰氏阴性细菌均无活性。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号