当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Divergent Syntheses of (+)-Bulleyanaline and Related Isoquinoline Alkaloids from the Genus Corydalis
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-09-16 , DOI: 10.1021/jacs.9b08441 Barry M Trost 1 , Chao-I Joey Hung 1 , Zhiwei Jiao 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-09-16 , DOI: 10.1021/jacs.9b08441 Barry M Trost 1 , Chao-I Joey Hung 1 , Zhiwei Jiao 1
Affiliation
|
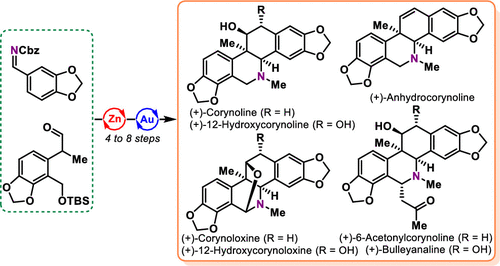
The isoquinoline alkaloids isolated from the genus Corydalis possess potent and diverse biological activities. Herein, a concise, divergent, and enantioselective route to access these natural products is disclosed. Key transformations of our approach include a challenging Zn-ProPhenol catalyzed asymmetric Mannich reaction to build a quaternary stereogenic center and a rapid cationic Au-catalyzed cycloisomerization to the common structural skeleton of these natural products. Subsequent late-stage oxidations and modifications allow efficient access to the targeted alkaloids. Overall, 7 natural products have been successfully synthesized in 6 to 10 steps from readily available starting materials, including (+)-corynoline, (+)-anhydrocorynoline, (+)-12-hydroxycorynoline, (+)-12-hydroxycorynoloxine, (+)-corynoloxine, (+)-6-acetonylcorynoline, and (+)-bulleyanaline.
中文翻译:

延胡索属 (+)-Bulleyanaline 和相关异喹啉生物碱的对映选择性发散合成
从延胡索属中分离出的异喹啉生物碱具有强大而多样的生物活性。在此,公开了获取这些天然产物的简明、发散和对映选择性途径。我们方法的关键转变包括具有挑战性的 Zn-ProPhenol 催化的不对称曼尼希反应以构建四元立体中心和快速阳离子 Au 催化的环异构化到这些天然产物的共同结构骨架。随后的后期氧化和修饰可以有效地获取目标生物碱。总体而言,从易得的起始材料中,通过 6 到 10 步成功合成了 7 种天然产物,包括 (+)-corynoline、(+)-anhydrocorynoline、(+)-12-hydroxycorynoline、(+)-12-hydroxycorynoline,( +)-棒碱,(+)-6-乙酰基棒碱,
更新日期:2019-09-16
中文翻译:

延胡索属 (+)-Bulleyanaline 和相关异喹啉生物碱的对映选择性发散合成
从延胡索属中分离出的异喹啉生物碱具有强大而多样的生物活性。在此,公开了获取这些天然产物的简明、发散和对映选择性途径。我们方法的关键转变包括具有挑战性的 Zn-ProPhenol 催化的不对称曼尼希反应以构建四元立体中心和快速阳离子 Au 催化的环异构化到这些天然产物的共同结构骨架。随后的后期氧化和修饰可以有效地获取目标生物碱。总体而言,从易得的起始材料中,通过 6 到 10 步成功合成了 7 种天然产物,包括 (+)-corynoline、(+)-anhydrocorynoline、(+)-12-hydroxycorynoline、(+)-12-hydroxycorynoline,( +)-棒碱,(+)-6-乙酰基棒碱,











































 京公网安备 11010802027423号
京公网安备 11010802027423号