Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2019-09-02 , DOI: 10.1016/j.molliq.2019.111661 A. Panuszko , P. Bruździak , M. Śmiechowski , M. Stasiulewicz , J. Stefaniak , J. Stangret
|
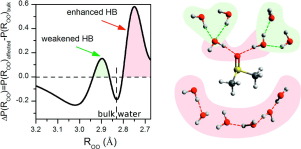
具有混合的亲水-疏水特性的溶质的疏水水合仍然知之甚少。这是因为,无论是实验方法还是理论方法,都很难看到非极性溶质基团周围的冰状水结构,这与带有亲水基团的氢键不同。为了解决这个问题,我们通过红外光谱和理论方法(即DFT,ONIOM计算和AIMD模拟)研究了DMSO的水合作用,从而重新定义了其水合作用。在稀释的DMSO溶液中,在水分子与甲基氢(蓝移氢键)的相互作用下,在DMSO分子周围形成了包合物状水。笼子是由水分子构成的,这些水分子形成的能量和长度与SO基团和水分子之间具有可比的氢键。笼子的构造完成后,DMSO分子会在内部部分恢复其旋转自由度。框架内的强氢键被SO基团附近相对较小的水分子氢键弱化所掩盖,这是由于与DMSO氧原子键合的水分子氢的配位不当所致。我们还提出了对DMSO水溶液在共晶点高度高度不理想的混合行为的新解释,因为该体系中等摩尔量的分子配合物具有正过量熵。DMSO分子内部部分恢复了旋转自由度。框架内的强氢键被SO基团附近相对较小的水分子氢键弱化所掩盖,这是由于与DMSO氧原子键合的水分子氢的配位不当所致。我们还提出了对DMSO水溶液在共晶点高度非理想混合行为的新解释,因为该体系中分子复合物的等摩尔量的正过量熵。DMSO分子内部部分恢复了旋转自由度。框架内的强氢键被SO基团附近相对较小的水分子氢键弱化所掩盖,这是由于与DMSO氧原子键合的水分子氢的配位不当所致。我们还提出了对DMSO水溶液在共晶点高度非理想混合行为的新解释,因为该体系中分子复合物的等摩尔量的正过量熵。由于与大量水分子的不合适配合,氢分子与DMSO的氧原子键合。我们还提出了对DMSO水溶液在共晶点高度非理想混合行为的新解释,因为该体系中分子复合物的等摩尔量的正过量熵。由于与大量水分子的不合适配合,氢分子与DMSO的氧原子键合。我们还提出了对DMSO水溶液在共晶点高度非理想混合行为的新解释,因为该体系中分子复合物的等摩尔量的正过量熵。

"点击查看英文标题和摘要"


















































 京公网安备 11010802027423号
京公网安备 11010802027423号