Synthesis ( IF 2.2 ) Pub Date : 2019-07-30 , DOI: 10.1055/s-0039-1690016 Pablo Cortón , Paula Novo , Vanesa López-Sobrado , Marcos D. García , Carlos Peinador , Elena Pazos 1
|
Published as part of the Bürgenstock Special Section 2019 Future Stars in Organic Chemistry
Abstract
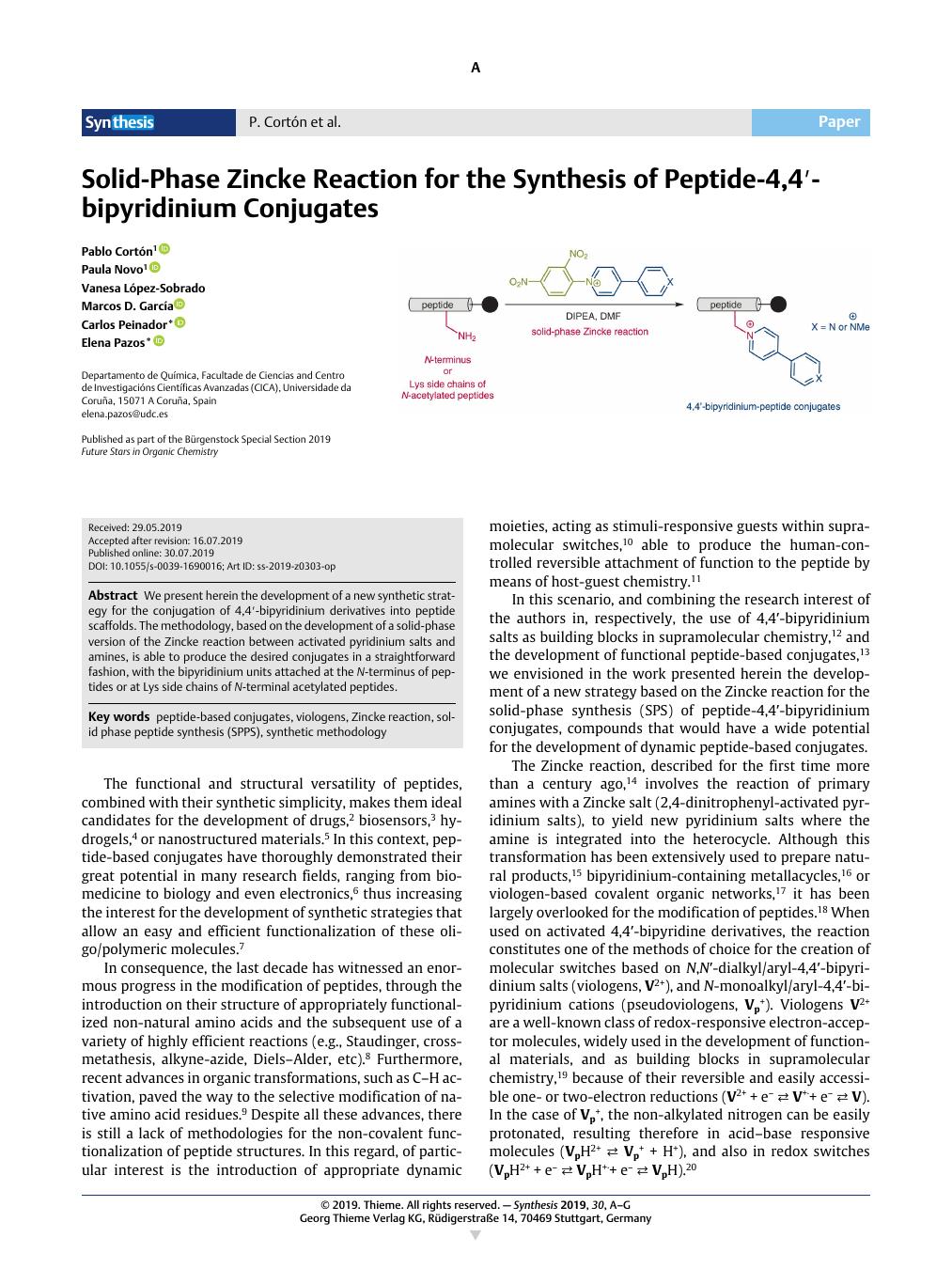
We present herein the development of a new synthetic strategy for the conjugation of 4,4′-bipyridinium derivatives into peptide scaffolds. The methodology, based on the development of a solid-phase version of the Zincke reaction between activated pyridinium salts and amines, is able to produce the desired conjugates in a straightforward fashion, with the bipyridinium units attached at the N-terminus of peptides or at Lys side chains of N-terminal acetylated peptides.
We present herein the development of a new synthetic strategy for the conjugation of 4,4′-bipyridinium derivatives into peptide scaffolds. The methodology, based on the development of a solid-phase version of the Zincke reaction between activated pyridinium salts and amines, is able to produce the desired conjugates in a straightforward fashion, with the bipyridinium units attached at the N-terminus of peptides or at Lys side chains of N-terminal acetylated peptides.
中文翻译:

固相Zincke反应合成4,4'-联吡啶共轭肽
作为Bürgenstock特别版2019年有机化学中的未来之星的一部分发布
抽象的
我们在本文中提出了将4,4'-联吡啶衍生物缀合到肽支架中的新合成策略的开发。该方法基于活化吡啶鎓盐和胺之间的Zincke反应的固相形式的开发,能够以简单的方式产生所需的偶联物,联吡啶鎓单元连接在肽的N端或N末端乙酰化肽的Lys侧链。
我们在本文中提出了将4,4'-联吡啶衍生物缀合到肽支架中的新合成策略的开发。该方法基于活化吡啶鎓盐和胺之间的Zincke反应的固相形式的开发,能够以简单的方式产生所需的偶联物,联吡啶鎓单元连接在肽的N端或N末端乙酰化肽的Lys侧链。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号