当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Local Structure of Supported Keggin and Wells–Dawson Heteropolyacids and Its Influence on the Catalytic Activity
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-08-02 , DOI: 10.1021/acs.jpcc.9b03659 Elisa I. García-López , Giuseppe Marcì , Igor Krivtsov 1 , Jorge Casado Espina , Leonarda F. Liotta 2 , Aida Serrano 3
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-08-02 , DOI: 10.1021/acs.jpcc.9b03659 Elisa I. García-López , Giuseppe Marcì , Igor Krivtsov 1 , Jorge Casado Espina , Leonarda F. Liotta 2 , Aida Serrano 3
Affiliation
|
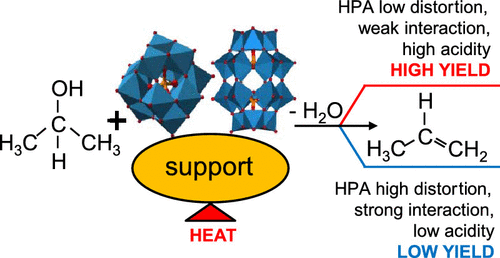
Keggin [PW12O40]3– and Wells–Dawson [P2W18O62]6– heteropolyanions are nanosized transition-metal-oxygen clusters belonging to the heteropolyacids (HPAs) family. They are widely used as catalysts due to their high Brønsted acidity, and their dispersion on solid supports favors the accessibility to their acid sites generally increasing the catalytic activity. A series of binary materials composed of Keggin or Wells–Dawson HPAs and SiO2, TiO2, and ZrO2 have been prepared by impregnation or solvothermal methods. Remarkable differences have been found in the catalytic activities among the unsupported and supported HPAs. These differences have been correlated in the past to the structural changes of the HPAs due to the cluster–support interaction, which is different depending on preparation methodologies of the binary material. In the present work, the modes of interaction between the two types of HPA, Keggin and Wells–Dawson, and various supports have been studied by X-ray absorption spectroscopy. The obtained data have been compared to the characterization of the same materials reported before by using different bulk and surface physicochemical techniques. The characterization results were then used to correlate the interaction modes between the HPAs and the supports with the catalytic performances reported for 2-propanol dehydration to propene and for propene hydration to 2-propanol. The results reveal that the deposition of HPA by impregnation or solvothermal treatment may cause distortions in the H3PW12O40 cluster structure depending on the presence of stronger (TiO2 and ZrO2) or weaker (SiO2) basic sites in the support, respectively. Moreover, the type of preparation method affects the structure and acidic properties of the supported HPAs. In particular, during the preparation of TiO2 and ZrO2 with HPA by in situ solvothermal method, the reaction of the HPA with the products of metal alkoxides hydrolysis occurs with consequent destruction of the Keggin structure. Therefore, the catalytic activity of such materials is poor. These modifications, in addition to the bulk and surface features of the supported HPAs, affected the catalytic 2-propanol dehydration to a significant extent. On the contrary, the propene hydration was less influenced, probably, due to the propene nonpolar nature.
中文翻译:

负载的Keggin和Wells-Dawson杂多酸的局部结构及其对催化活性的影响
Keggin [PW 12 O 40 ] 3 –和Wells–Dawson [P 2 W 18 O 62 ] 6 –杂多阴离子是属于杂多酸(HPA)家族的纳米级过渡金属-氧簇。由于它们的高布朗斯台德酸度,它们被广泛用作催化剂,并且它们在固体载体上的分散有利于其酸点的可及性,从而通常提高了催化活性。一系列由Keggin或Wells-Dawson HPA和SiO 2,TiO 2和ZrO 2组成的二元材料已经通过浸渍或溶剂热法制备。在未得到支持和得到支持的HPA之间,在催化活性方面发现了显着差异。过去,由于簇-载体相互作用,这些差异与HPA的结构变化相关,而这种变化取决于二元材料的制备方法而有所不同。在目前的工作中,已经通过X射线吸收光谱研究了HPA的两种类型Keggin和Wells-Dawson以及各种支持物之间的相互作用模式。通过使用不同的体积和表面物理化学技术,将获得的数据与以前报道的相同材料的特性进行了比较。然后将表征结果用于将HPA与载体之间的相互作用方式与报道的2-丙醇脱水成丙烯和丙烯水合成2-丙醇的催化性能相关联。结果表明,通过浸渍或溶剂热处理沉积的HPA可能会导致H的变形3 PW 12 O 40团簇结构分别取决于载体中存在较强的(TiO 2和ZrO 2)或较弱的(SiO 2)碱性位。而且,制备方法的类型会影响所支持的HPA的结构和酸性。特别是在制备TiO 2和ZrO 2的过程中用原位溶剂热法对HPA进行反应时,HPA与金属醇盐水解产物发生反应,从而破坏了Keggin结构。因此,这种材料的催化活性差。除了负载的HPA的体积和表面特征外,这些修饰在很大程度上影响了催化2-丙醇的脱水。相反,由于丙烯的非极性性质,丙烯水合的影响较小。
更新日期:2019-08-03
中文翻译:

负载的Keggin和Wells-Dawson杂多酸的局部结构及其对催化活性的影响
Keggin [PW 12 O 40 ] 3 –和Wells–Dawson [P 2 W 18 O 62 ] 6 –杂多阴离子是属于杂多酸(HPA)家族的纳米级过渡金属-氧簇。由于它们的高布朗斯台德酸度,它们被广泛用作催化剂,并且它们在固体载体上的分散有利于其酸点的可及性,从而通常提高了催化活性。一系列由Keggin或Wells-Dawson HPA和SiO 2,TiO 2和ZrO 2组成的二元材料已经通过浸渍或溶剂热法制备。在未得到支持和得到支持的HPA之间,在催化活性方面发现了显着差异。过去,由于簇-载体相互作用,这些差异与HPA的结构变化相关,而这种变化取决于二元材料的制备方法而有所不同。在目前的工作中,已经通过X射线吸收光谱研究了HPA的两种类型Keggin和Wells-Dawson以及各种支持物之间的相互作用模式。通过使用不同的体积和表面物理化学技术,将获得的数据与以前报道的相同材料的特性进行了比较。然后将表征结果用于将HPA与载体之间的相互作用方式与报道的2-丙醇脱水成丙烯和丙烯水合成2-丙醇的催化性能相关联。结果表明,通过浸渍或溶剂热处理沉积的HPA可能会导致H的变形3 PW 12 O 40团簇结构分别取决于载体中存在较强的(TiO 2和ZrO 2)或较弱的(SiO 2)碱性位。而且,制备方法的类型会影响所支持的HPA的结构和酸性。特别是在制备TiO 2和ZrO 2的过程中用原位溶剂热法对HPA进行反应时,HPA与金属醇盐水解产物发生反应,从而破坏了Keggin结构。因此,这种材料的催化活性差。除了负载的HPA的体积和表面特征外,这些修饰在很大程度上影响了催化2-丙醇的脱水。相反,由于丙烯的非极性性质,丙烯水合的影响较小。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号