当前位置:
X-MOL 学术
›
Chem. Eng. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Deposition Mechanism of Electroless Nickel Plating of Composite Coatings on Magnesium Alloy
Chemical Engineering Science ( IF 4.1 ) Pub Date : 2019-11-01 , DOI: 10.1016/j.ces.2019.07.048 Wei Shang , Xiaoqiang Zhan , Yuqing Wen , Yuqing Li , Zhe Zhang , Fang Wu , Chunlei Wang
Chemical Engineering Science ( IF 4.1 ) Pub Date : 2019-11-01 , DOI: 10.1016/j.ces.2019.07.048 Wei Shang , Xiaoqiang Zhan , Yuqing Wen , Yuqing Li , Zhe Zhang , Fang Wu , Chunlei Wang
|
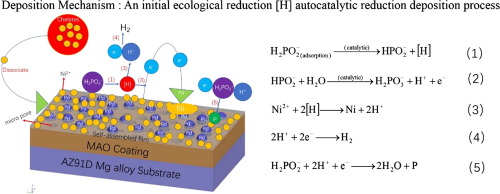
Abstract To investigate the deposition mechanism of the outermost electroless nickel plating layer in a three-layer composite coatings on the magnesium alloy surface, the electroless nickel plating layers with six different plating times (3, 5, 10, 30, 60, and 90 minutes) were selected as research targets. The transformations in microcosmic morphology, element composition and material structure of the samples electrolessly plated at six different plating times were characterized by SEM、EDS and XRD. The changes in corrosion resistance of samples with different electroless plating times were measured by polarization curve. It was concluded that the nickel-phosphorus deposition process on the activation composite coatings was a “cell-like three-dimensional” growth judging from the microscopic morphology of coating surface obtained by SEM. The elemental changes obtained from the EDS were consistent with the growth of nickel cells obtained SEM. The XRD results showed that the diffraction peaks of nickel were not detected on the surface at an electroless plating time of 3 minutes. Nickel peaks appeared after 5 minutes of electroless plating, and the nickel peak width broadened and the intensity increased as the electroless plating time increased. The corrosion resistance is greatly improved when the electroless plating is performed for 60 minutes due to the complete nickel coating without defects as shown in the SEM. It can be concluded that the deposition mechanism of electroless plating on the double-layer active surface is an initial ecological reduction [H] autocatalytic reduction deposition process according to the micromorphology and structure of the samples electrolessly plated at six different plating times.
中文翻译:

镁合金复合镀层化学镀镍的沉积机理
摘要 为研究镁合金表面三层复合镀层中最外层化学镀镍层的沉积机理,化学镀镍层在 6 种不同的电镀时间(3、5、10、30、60 和 90 min ) 被选为研究对象。采用SEM、EDS和XRD表征了6种不同电镀时间化学镀样品的微观形貌、元素组成和材料结构的转变。通过极化曲线测量不同化学镀时间样品的耐蚀性变化。从扫描电镜获得的涂层表面微观形貌判断,活化复合涂层上的镍磷沉积过程为“细胞状三维”生长。从 EDS 获得的元素变化与从 SEM 获得的镍电池的生长一致。XRD结果表明,在3分钟的化学镀时间下,在表面上没有检测到镍的衍射峰。化学镀5分钟后出现镍峰,随着化学镀时间的增加,镍峰宽度变宽,强度增加。当化学镀60分钟时,由于完整的镍镀层没有缺陷,如SEM所示,耐腐蚀性大大提高。
更新日期:2019-11-01
中文翻译:

镁合金复合镀层化学镀镍的沉积机理
摘要 为研究镁合金表面三层复合镀层中最外层化学镀镍层的沉积机理,化学镀镍层在 6 种不同的电镀时间(3、5、10、30、60 和 90 min ) 被选为研究对象。采用SEM、EDS和XRD表征了6种不同电镀时间化学镀样品的微观形貌、元素组成和材料结构的转变。通过极化曲线测量不同化学镀时间样品的耐蚀性变化。从扫描电镜获得的涂层表面微观形貌判断,活化复合涂层上的镍磷沉积过程为“细胞状三维”生长。从 EDS 获得的元素变化与从 SEM 获得的镍电池的生长一致。XRD结果表明,在3分钟的化学镀时间下,在表面上没有检测到镍的衍射峰。化学镀5分钟后出现镍峰,随着化学镀时间的增加,镍峰宽度变宽,强度增加。当化学镀60分钟时,由于完整的镍镀层没有缺陷,如SEM所示,耐腐蚀性大大提高。





























 京公网安备 11010802027423号
京公网安备 11010802027423号