Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2019-07-19 , DOI: 10.1016/j.bioorg.2019.103134 Fatoş Erdemir 1 , Duygu Barut Celepci 2 , Aydın Aktaş 1 , Yetkin Gök 1 , Ruya Kaya 3 , Parham Taslimi 4 , Yeliz Demir 5 , İlhami Gulçin 6
|
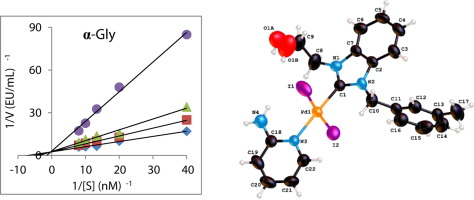
In this work, the synthesis, crystal structure, characterization, and enzyme inhibition effects of the novel a series of 2-aminopyridine liganded Pd(II) N-heterocyclic carbene (NHC) complexes were examined. These complexes of the Pd-based were synthesized from PEPPSI complexes and 2-aminopyridine. The novel complexes were characterized by using 13C NMR, 1H NMR, elemental analysis, and FTIR spectroscopy techniques. Also, crystal structures of the two compounds were recorded by using single-crystal X-ray diffraction assay. Also, these complexes were tested toward some metabolic enzymes like α-glycosidase, aldose reductase, butyrylcholinesterase, acetylcholinesterase enzymes, and carbonic anhydrase I, and II isoforms. The novel 2-aminopyridine liganded (NHC)PdI2(2-aminopyridine) complexes (1a-i) showed Ki values of in range of 5.78 ± 0.33–22.51 ± 8.59 nM against hCA I, 13.77 ± 2.21–30.81 ± 4.87 nM against hCA II, 0.44 ± 0.08–1.87 ± 0.11 nM against AChE and 3.25 ± 0.34–12.89 ± 4.77 nM against BChE. Additionally, we studied the inhibition effect of these derivatives on aldose reductase and α-glycosidase enzymes. For these compounds, compound 1d showed maximum inhibition effect against AR with a Ki value of 360.37 ± 55.82 nM. Finally, all compounds were tested for the inhibition of α-glycosidase enzyme, which recorded efficient inhibition profiles with Ki values in the range of 4.44 ± 0.65–12.67 ± 2.50 nM against α-glycosidase.
中文翻译:

新型2-氨基吡啶配体的Pd(II)N-杂环卡宾配合物:合成,表征,晶体结构和生物活性。
在这项工作中,合成,晶体结构,表征和酶抑制作用的新型2-氨基吡啶配体的Pd(II)N-杂环卡宾(NHC)配合物系列进行了审查。这些基于Pd的配合物是由PEPPSI配合物和2-氨基吡啶合成的。通过使用13 C NMR,1 H NMR,元素分析和FTIR光谱技术对新型配合物进行表征。另外,通过使用单晶X射线衍射测定法记录了两种化合物的晶体结构。此外,还针对某些代谢酶(例如α-糖苷酶,醛糖还原酶,丁酰胆碱酯酶,乙酰胆碱酯酶和碳酸酐酶I和II同工型)对这些复合物进行了测试。新型2-氨基吡啶配体(NHC)PdI2种(2-氨基吡啶)配合物(1a-i)对hCA I的Ki值范围为5.78±0.33–22.51±8.59 nM,对hCA II的Ki值范围为13.77±2.21–30.81±4.87 nM,0.44±0.08–1.87±0.11针对AChE的nM和针对BChE的3.25±0.34–12.89±4.77 nM。另外,我们研究了这些衍生物对醛糖还原酶和α-糖苷酶的抑制作用。对于这些化合物,化合物1d对AR的抑制作用最大,Ki值为360.37±55.82 nM。最后,测试了所有化合物对α-糖苷酶的抑制作用,该酶记录了对α-糖苷酶的有效抑制谱,Ki值在4.44±0.65–12.67±2.50 nM范围内。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号