当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Polypeptide-engineered DNA tetrahedrons for targeting treatment of colorectal cancer via apoptosis and autophagy.
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2019-07-10 , DOI: 10.1016/j.jconrel.2019.07.012 Nan Zhang 1 , Yanan Yang 2 , Ziyi Wang 2 , Jing Yang 2 , Xiao Chu 2 , Jin Liu 3 , Yongxing Zhao 1
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2019-07-10 , DOI: 10.1016/j.jconrel.2019.07.012 Nan Zhang 1 , Yanan Yang 2 , Ziyi Wang 2 , Jing Yang 2 , Xiao Chu 2 , Jin Liu 3 , Yongxing Zhao 1
Affiliation
|
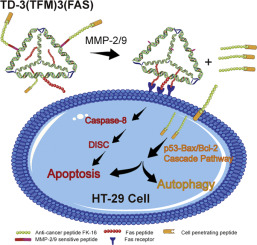
Smart delivery of therapeutic peptides that target cellular signaling pathways holds high specificity and great promise for cancer therapy. Here, DNA tetrahedrons (TDs) are designed to carry two therapeutic peptides-FAS peptide and FK-16 peptide. DNA TDs are designed with varied numbers and spatial placement of FAS peptides and FK-16 peptides, and tested for their anti-cancer efficacy. Trimerization of FAS receptors using TDs that are assembled with three FAS peptides enhances FAS-induced cell apoptosis. FK-16 peptides are conjugated to TDs via a peptide sequence sensitive to MMP-2/9 in tumor microenvironment. Therefore, FK-16 peptides are expected to detach from TDs once arrived the tumor microenvironment. A cell penetrating peptide (TAT) is also conjugated to the FK-16 peptide to facilitate its intracellular delivery, which increases the FK-16 peptide-induced cell apoptosis and autophagy. TD-3(TFM)3(FAS) (TFM: TAT + FK-16 + MMP-2/9) exhibits the highest HT-29 inhibition in vitro and in vivo among all therapies. In addition to the high anti-cancer efficacy, TD-3(TFM)3(FAS) shows a high specificity to HT-29 cells in vitro and in vivo. Low cell inhibition rates and cellular uptake are observed in normal cells. In sum, the multifunctional TD-3(TFM)3(FAS) provides a new platform for the smart delivery of anti-cancer peptides to achieve enhanced efficacy and high specificity.
中文翻译:

多肽工程化的DNA四面体可通过凋亡和自噬靶向治疗结直肠癌。
靶向细胞信号传导途径的治疗性肽的智能传递具有高度特异性,对癌症治疗具有广阔的前景。在这里,DNA四面体(TDs)被设计为携带两个治疗性肽-FAS肽和FK-16肽。设计了具有不同数量和空间位置的FAS肽和FK-16肽的DNA TD,并对其抗癌功效进行了测试。使用与三种FAS肽组装的TD对FAS受体进行三聚化可增强FAS诱导的细胞凋亡。FK-16肽通过对肿瘤微环境中MMP-2 / 9敏感的肽序列与TD偶联。因此,一旦到达肿瘤微环境,FK-16肽有望与TD分离。细胞穿透肽(TAT)也与FK-16肽缀合,以促进其细胞内递送,这会增加FK-16肽诱导的细胞凋亡和自噬。在所有疗法中,TD-3(TFM)3(FAS)(TFM:TAT + FK-16 + MMP-2 / 9)在体外和体内均表现出最高的HT-29抑制作用。TD-3(TFM)3(FAS)除具有很高的抗癌功效外,在体外和体内均对HT-29细胞具有高度特异性。在正常细胞中观察到低的细胞抑制率和细胞摄取。总之,多功能TD-3(TFM)3(FAS)为智能递送抗癌肽提供了一个新平台,以实现增强的功效和高特异性。在正常细胞中观察到低的细胞抑制率和细胞摄取。总之,多功能TD-3(TFM)3(FAS)为智能递送抗癌肽提供了一个新平台,以实现增强的功效和高特异性。在正常细胞中观察到低的细胞抑制率和细胞摄取。总之,多功能TD-3(TFM)3(FAS)为智能递送抗癌肽提供了一个新平台,以实现增强的功效和高特异性。
更新日期:2019-07-10
中文翻译:

多肽工程化的DNA四面体可通过凋亡和自噬靶向治疗结直肠癌。
靶向细胞信号传导途径的治疗性肽的智能传递具有高度特异性,对癌症治疗具有广阔的前景。在这里,DNA四面体(TDs)被设计为携带两个治疗性肽-FAS肽和FK-16肽。设计了具有不同数量和空间位置的FAS肽和FK-16肽的DNA TD,并对其抗癌功效进行了测试。使用与三种FAS肽组装的TD对FAS受体进行三聚化可增强FAS诱导的细胞凋亡。FK-16肽通过对肿瘤微环境中MMP-2 / 9敏感的肽序列与TD偶联。因此,一旦到达肿瘤微环境,FK-16肽有望与TD分离。细胞穿透肽(TAT)也与FK-16肽缀合,以促进其细胞内递送,这会增加FK-16肽诱导的细胞凋亡和自噬。在所有疗法中,TD-3(TFM)3(FAS)(TFM:TAT + FK-16 + MMP-2 / 9)在体外和体内均表现出最高的HT-29抑制作用。TD-3(TFM)3(FAS)除具有很高的抗癌功效外,在体外和体内均对HT-29细胞具有高度特异性。在正常细胞中观察到低的细胞抑制率和细胞摄取。总之,多功能TD-3(TFM)3(FAS)为智能递送抗癌肽提供了一个新平台,以实现增强的功效和高特异性。在正常细胞中观察到低的细胞抑制率和细胞摄取。总之,多功能TD-3(TFM)3(FAS)为智能递送抗癌肽提供了一个新平台,以实现增强的功效和高特异性。在正常细胞中观察到低的细胞抑制率和细胞摄取。总之,多功能TD-3(TFM)3(FAS)为智能递送抗癌肽提供了一个新平台,以实现增强的功效和高特异性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号