Nature Communications ( IF 14.7 ) Pub Date : 2019-06-13 , DOI: 10.1038/s41467-019-10496-3 Futang Wan 1, 2, 3 , Qianmin Wang 4, 5 , Jing Tan 1, 2 , Ming Tan 1, 2 , Juan Chen 4, 5 , Shaohua Shi 4, 5 , Pengfei Lan 4, 5 , Jian Wu 4, 5, 6 , Ming Lei 4, 5, 7
|
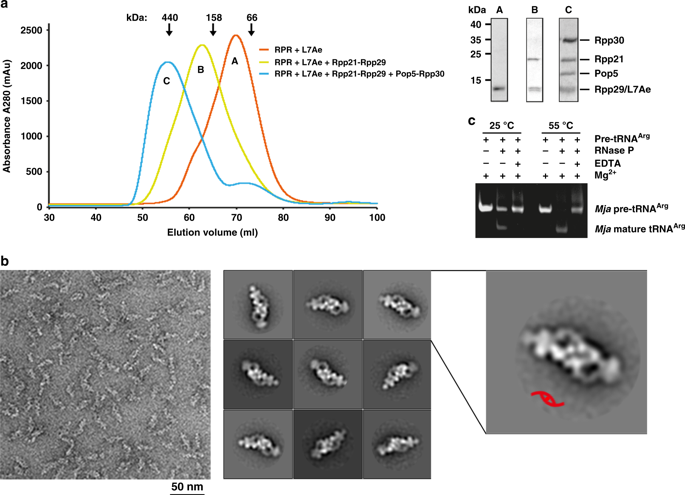
Ribonuclease P (RNase P) is an essential ribozyme responsible for tRNA 5′ maturation. Here we report the cryo-EM structures of Methanocaldococcus jannaschii (Mja) RNase P holoenzyme alone and in complex with a tRNA substrate at resolutions of 4.6 Å and 4.3 Å, respectively. The structures reveal that the subunits of MjaRNase P are strung together to organize the holoenzyme in a dimeric conformation required for efficient catalysis. The structures also show that archaeal RNase P is a functional chimera of bacterial and eukaryal RNase Ps that possesses bacterial-like two RNA-based anchors and a eukaryal-like protein-aided stabilization mechanism. The 3′-RCCA sequence of tRNA, which is a key recognition element for bacterial RNase P, is dispensable for tRNA recognition by MjaRNase P. The overall organization of MjaRNase P, particularly within the active site, is similar to those of bacterial and eukaryal RNase Ps, suggesting a universal catalytic mechanism for all RNase Ps.
中文翻译:

古细菌核糖核酸酶P全酶的低温电子显微镜结构。
核糖核酸酶P(RNase P)是负责tRNA 5'成熟的必需核酶。在这里,我们报告单独和与tRNA底物复杂的甲烷甲烷球菌(Mja)RNase P全酶的冷冻EM结构,分别为4.6Å和4.3Å的分辨率。结构揭示了Mja的亚基将RNase P串在一起,以有效催化所需的二聚体构象组织全酶。该结构还表明,古细菌RNase P是细菌和真核RNase Ps的功能嵌合体,具有细菌样的两个基于RNA的锚点和真核样的蛋白质辅助稳定机制。的tRNA,其是用于细菌核糖核酸酶P的关键识别元件的3'- RCCA序列,是可有可无的tRNA的识别通过MJA核糖核酸酶P的整体组织MJA核糖核酸酶P,特别是在活性位点,类似于那些细菌和真核RNase Ps,暗示了所有RNase Ps的普遍催化机制。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号