当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Atomic-Level Insight into the Postsynthesis Band Gap Engineering of a Lewis Base Polymer Using Lewis Acid Tris(pentafluorophenyl)borane
Chemistry of Materials ( IF 7.2 ) Pub Date : 2019-05-15 00:00:00 , DOI: 10.1021/acs.chemmater.9b01224 Brett Yurash , Dirk Leifert , G. N. Manjunatha Reddy , David Xi Cao , Simon Biberger 1 , Viktor V. Brus , Martin Seifrid , Peter J. Santiago , Anna Köhler 1 , Bradley F. Chmelka , Guillermo C. Bazan , Thuc-Quyen Nguyen
Chemistry of Materials ( IF 7.2 ) Pub Date : 2019-05-15 00:00:00 , DOI: 10.1021/acs.chemmater.9b01224 Brett Yurash , Dirk Leifert , G. N. Manjunatha Reddy , David Xi Cao , Simon Biberger 1 , Viktor V. Brus , Martin Seifrid , Peter J. Santiago , Anna Köhler 1 , Bradley F. Chmelka , Guillermo C. Bazan , Thuc-Quyen Nguyen
Affiliation
|
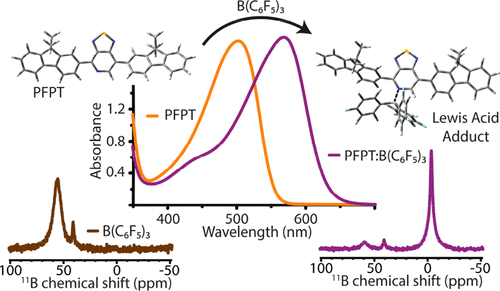
In this report, we investigate the binding properties of the Lewis acid tris(pentafluorophenyl)borane with a Lewis base semiconducting polymer, PFPT, and the subsequent mechanism of band gap reduction. Experiments and quantum chemical calculations confirm that the formation of a Lewis acid adduct is energetically favorable (ΔG° < −0.2 eV), with preferential binding at the pyridyl nitrogen in the polymer backbone over other Lewis base sites. Upon adduct formation, ultraviolet photoelectron spectroscopy indicates only a slight decrease in the HOMO energy, implying that a larger reduction in the LUMO energy is primarily responsible for the observed optical band gap narrowing (ΔEopt = 0.3 eV). Herein, we also provide the first spatially resolved picture of how Lewis acid adducts form in heterogeneous, disordered polymer/tris(pentafluorophenyl)borane thin films via one- (1D) and two-dimensional (2D) solid-state nuclear magnetic resonance. Notably, solid-state 1D 11B, 13C{1H}, and 13C{19F} cross-polarization magic-angle spinning (CP-MAS) NMR and 2D 1H{19F} and 1H{1H} correlation NMR analyses establish that BCF molecules are intercalated between branched C16H33 side chains with the boron atom facing toward the pyridyl nitrogen atoms of PFPT.
中文翻译:

使用路易斯酸三(五氟苯基)硼烷的路易斯基础聚合物的合成后带隙工程的原子级洞察力
在本报告中,我们研究了路易斯酸三(五氟苯基)硼烷与路易斯碱半导体聚合物PFPT的结合性能,以及随后的带隙减小机理。实验和量子化学计算证实,路易斯酸加合物的形成在能量上是有利的(ΔG °<-0.2 eV),与其他路易斯碱位点相比,聚合物主链中的吡啶氮优先结合。形成加合物后,紫外光电子能谱表明HOMO能量仅略有降低,这意味着LUMO能量的较大降低是观察到的光学带隙变窄的主要原因(ΔE opt= 0.3 eV)。在本文中,我们还提供了关于路易斯酸加合物如何通过一维(1D)和二维(2D)固态核磁共振在非均相,无序聚合物/三(五氟苯基)硼烷薄膜中形成的路易斯酸加成物的第一张空间分辨图片。值得注意的是,固态1D 11 B,13 C { 1 H}和13 C { 19 F}交叉极化幻角旋转(CP-MAS)NMR和2D 1 H { 19 F}和1 H { 1 H }相关NMR分析表明,BCF分子插入在支链C 16 H 33之间 侧链,硼原子朝向PFPT的吡啶基氮原子。
更新日期:2019-05-15
中文翻译:

使用路易斯酸三(五氟苯基)硼烷的路易斯基础聚合物的合成后带隙工程的原子级洞察力
在本报告中,我们研究了路易斯酸三(五氟苯基)硼烷与路易斯碱半导体聚合物PFPT的结合性能,以及随后的带隙减小机理。实验和量子化学计算证实,路易斯酸加合物的形成在能量上是有利的(ΔG °<-0.2 eV),与其他路易斯碱位点相比,聚合物主链中的吡啶氮优先结合。形成加合物后,紫外光电子能谱表明HOMO能量仅略有降低,这意味着LUMO能量的较大降低是观察到的光学带隙变窄的主要原因(ΔE opt= 0.3 eV)。在本文中,我们还提供了关于路易斯酸加合物如何通过一维(1D)和二维(2D)固态核磁共振在非均相,无序聚合物/三(五氟苯基)硼烷薄膜中形成的路易斯酸加成物的第一张空间分辨图片。值得注意的是,固态1D 11 B,13 C { 1 H}和13 C { 19 F}交叉极化幻角旋转(CP-MAS)NMR和2D 1 H { 19 F}和1 H { 1 H }相关NMR分析表明,BCF分子插入在支链C 16 H 33之间 侧链,硼原子朝向PFPT的吡啶基氮原子。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号