Chem ( IF 19.1 ) Pub Date : 2019-05-02 , DOI: 10.1016/j.chempr.2019.04.023 Benke Hong , Weilong Liu , Jin Wang , Jinbao Wu , Yuichiro Kadonaga , Pei-Jun Cai , Hong-Xiang Lou , Zhi-Xiang Yu , Houhua Li , Xiaoguang Lei
|
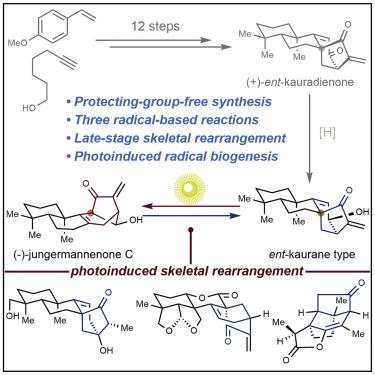
Herein, we describe the protecting-group-free total synthesis of two structurally diverse Isodon diterpenoids, (+)-ent-kauradienone (3) and (−)-jungermannenone C (4), in 12 and 14 steps respectively, through sequential applications of three radical-based reactions, including the photoinduced skeletal rearrangements of bicyclo[3.2.1]octene ring systems. Further investigations of this photochemical radical rearrangement on a series of diverse terpenoids demonstrated both the unparalleled functional-group tolerance and the broad applicability of such late-stage photochemical rearrangements for the synthesis of structurally diverse and complex small molecules. Overall, the mild nature of late-stage photoinduced skeletal rearrangements might suggest that they are possible in a biological setting in unappreciated complimentary biosynthetic pathways.
中文翻译:

光诱导的骨骼重排揭示了萜类化合物的自由基介导的合成。
在本文中,我们描述了两种结构多样的Isodon二萜类化合物(+)- ent- kauradienone(3)和(-)-jungermannenone C(4)的无保护基的全合成。)分别通过12和14个步骤,依次应用三个基于自由基的反应,包括双环[3.2.1]辛烯环系统的光诱导骨架重排。在一系列不同的萜类化合物上进行的光化学自由基重排的进一步研究表明,无与伦比的官能团耐受性以及此类后期光化学重排在合成结构多样和复杂的小分子方面的广泛适用性。总体而言,晚期光诱导性骨骼重排的温和性质可能表明它们可能在生物学环境中以未意识到的互补生物合成途径出现。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号