当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transition‐Metal‐Free Carbonylative Suzuki‐Miyaura Reactions of Aryl Iodides with Arylboronic Acids Using N‐Formylsaccharin as CO Surrogate
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-05-09 , DOI: 10.1002/adsc.201900306 Dezhong Yu 1 , Fangning Xu 2 , Dan Li 3 , Wei Han 2, 3
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-05-09 , DOI: 10.1002/adsc.201900306 Dezhong Yu 1 , Fangning Xu 2 , Dan Li 3 , Wei Han 2, 3
Affiliation
|
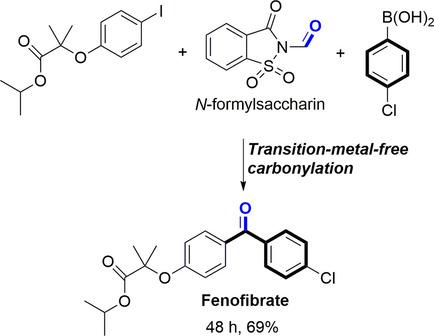
Unprecedented, high yielding, transition‐metal‐free carbonylative Suzuki‐Miyaura reactions of aryl iodides with arylboronic acids using N‐formylsaccharin as CO surrogate have been developed. Notably, this general protocol was adapted to the synthesis of the triglyceride and cholesterol regulator drug, fenofibrate, and carbon‐13 labeled biaryl ketone.
中文翻译:

使用N-甲酰基糖精作为CO替代物的芳基碘化物与芳基硼酸的无过渡金属羰基化Suzuki-Miyaura反应
使用N-甲酰基糖精作为CO替代物,开发了空前的,高收率,无过渡金属的羰基Suzuki-Miyaura芳基碘化物与芳基硼酸反应。值得注意的是,该通用方案适用于甘油三酸酯和胆固醇调节剂非诺贝特和碳13标记的联芳基酮的合成。
更新日期:2019-05-09
中文翻译:

使用N-甲酰基糖精作为CO替代物的芳基碘化物与芳基硼酸的无过渡金属羰基化Suzuki-Miyaura反应
使用N-甲酰基糖精作为CO替代物,开发了空前的,高收率,无过渡金属的羰基Suzuki-Miyaura芳基碘化物与芳基硼酸反应。值得注意的是,该通用方案适用于甘油三酸酯和胆固醇调节剂非诺贝特和碳13标记的联芳基酮的合成。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号