Catalysis Communications ( IF 3.4 ) Pub Date : 2019-04-04 , DOI: 10.1016/j.catcom.2019.04.005 Kittisak Choojun , Arucha Worathanaseth , Satu Kuhatasanadeekul , Teeraporn Kurato , Supanut Ketaniruj , Ploynisa Phichitsurathaworn , Pratya Promchana , Kittipong Prakobtham , Natthida Numwong , Yingyot Poo-arporn , Tawan Sooknoi
|
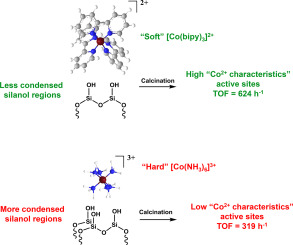
Effect of the cobalt precursors, including [Co(bipy)3](NO3)2, [Co(NH3)5Cl]Cl2, [Co(NH3)6]Cl3, and [Co(en)2Cl2] Cl, on reactivity of the cationic Co/SiO2 prepared by strong electrostatic adsorption (SEA) was investigated for the dehydrogenation of cyclohexane as a model reaction. According to the charge density of the cobalt complex, highly dispersed Co2+ species and/or Co3+ oxide can be obtained on the silica surface. The dehydrogenation activity is in the order of Co/SiO2 catalysts prepared by [Co(bipy)3](NO3)2 > [Co(NH3)5Cl]Cl2 > [Co(NH3)6]Cl3 > [Co(en)2Cl2] Cl, correlating to the Co2+ content of the final catalysts. The cationic cobalt catalysts are more active than the pre-reduced one. Although metallic cobalt is found to be less active, the activity of cationic cobalt catalyst is enhanced under H2 flow, presumably due to the formation of cobalt hydride intermediate. The inter-conversion of Co2+/cobalt hydride intermediate is readily reversible and regulated by presence of hydrogen.
中文翻译:

钴配合物前体对阳离子钴催化剂反应活性的影响:环己烷脱氢
钴前体的作用,包括[Co(bipy)3 ](NO 3)2,[Co(NH 3)5 Cl] Cl 2,[Co(NH 3)6 ] Cl 3和[Co(en)2以Cl 2 ] Cl为例,研究了强静电吸附(SEA)制得的阳离子Co / SiO 2对环己烷脱氢反应的反应性。根据钴配合物的电荷密度,可以在二氧化硅表面上获得高度分散的Co 2+物种和/或Co 3+氧化物。脱氢活性约为Co / SiO通过[Co(bipy)3 ](NO 3)2 > [Co(NH 3)5 Cl] Cl 2 > [Co(NH 3)6 ] Cl 3 > [Co(en)2 Cl 2 ] Cl制备2种催化剂与最终催化剂的Co 2+含量相关。阳离子钴催化剂比预还原的催化剂活性更高。尽管发现金属钴的活性较低,但推测是由于氢化钴中间体的形成,使得阳离子钴催化剂的活性在H 2流动下得以增强。Co 2+的互变/氢化钴中间体易于逆转并受氢的存在调节。





























 京公网安备 11010802027423号
京公网安备 11010802027423号