当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
π–π Stacking Interaction in an Oxidized CuII–Salen Complex with a Side‐Chain Indole Ring: An Approach to the Function of the Tryptophan in the Active Site of Galactose Oxidase
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2019-05-13 , DOI: 10.1002/chem.201900733 Hiromi Oshita 1, 2 , Takashi Suzuki 1 , Kyohei Kawashima 1 , Hitoshi Abe 3 , Fumito Tani 4 , Seiji Mori 1 , Tatsuo Yajima 5 , Yuichi Shimazaki 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2019-05-13 , DOI: 10.1002/chem.201900733 Hiromi Oshita 1, 2 , Takashi Suzuki 1 , Kyohei Kawashima 1 , Hitoshi Abe 3 , Fumito Tani 4 , Seiji Mori 1 , Tatsuo Yajima 5 , Yuichi Shimazaki 1
Affiliation
|
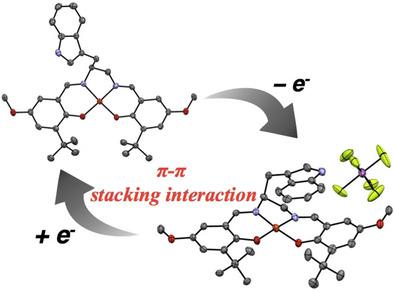
In order to gain new insights into the effect of the π–π stacking interaction of the indole ring with the CuII–phenoxyl radical as seen in the active form of galactose oxidase, we have prepared a CuII complex of a methoxy‐substituted salen‐type ligand, containing a pendent indole ring on the dinitrogen chelate backbone, and characterized its one‐electron‐oxidized forms. The X‐ray crystal structures of the oxidized CuII complex exhibited the π–π stacking interaction of the indole ring mainly with one of the two phenolate moieties. The phenolate moiety in close contact with the indole moiety showed the characteristic phenoxyl radical structural features, indicating that the indole ring favors the π–π stacking interaction with the phenoxyl radical. The UV/Vis/NIR spectra of the oxidized CuII complex with the pendent indole ring was significantly different from those of the complex without the side‐chain indole ring, and the absorption and CD spectra exhibited a solvent dependence, which is in line with the phenoxyl radical–indole stacking interaction in solution. The other physicochemical results and theoretical calculations strongly support that the indole ring, as an electron donor, stabilizes the phenoxyl radical by the π–π stacking interaction.
中文翻译:

带有侧链吲哚环的氧化CuII–Salen配合物中的π–π堆积相互作用:半乳糖氧化酶活性位点中色氨酸功能的途径
为了获得对以半乳糖氧化酶活性形式观察到的吲哚环与Cu II-苯氧基自由基的π-π堆积相互作用的影响的新见解,我们制备了甲氧基取代的salen的Cu II配合物型配体,在二氮螯合物主链上带有一个悬垂的吲哚环,并表征了其单电子氧化形式。氧化的铜II的X射线晶体结构配合物主要表现出吲哚环与两个酚盐部分之一的π-π堆积相互作用。与吲哚部分紧密接触的酚盐部分显示出特征性的苯氧基自由基结构特征,表明吲哚环有利于与苯氧基自由基的π-π堆积相互作用。氧化的铜II的UV / Vis / NIR光谱带有悬垂吲哚环的配合物与没有侧链吲哚环的配合物显着不同,并且吸收光谱和CD光谱表现出对溶剂的依赖性,这与溶液中苯氧基自由基-吲哚的堆积相互作用相符。其他物理化学结果和理论计算结果强烈支持吲哚环作为电子供体通过π-π堆积相互作用稳定苯氧基。
更新日期:2019-05-13
中文翻译:

带有侧链吲哚环的氧化CuII–Salen配合物中的π–π堆积相互作用:半乳糖氧化酶活性位点中色氨酸功能的途径
为了获得对以半乳糖氧化酶活性形式观察到的吲哚环与Cu II-苯氧基自由基的π-π堆积相互作用的影响的新见解,我们制备了甲氧基取代的salen的Cu II配合物型配体,在二氮螯合物主链上带有一个悬垂的吲哚环,并表征了其单电子氧化形式。氧化的铜II的X射线晶体结构配合物主要表现出吲哚环与两个酚盐部分之一的π-π堆积相互作用。与吲哚部分紧密接触的酚盐部分显示出特征性的苯氧基自由基结构特征,表明吲哚环有利于与苯氧基自由基的π-π堆积相互作用。氧化的铜II的UV / Vis / NIR光谱带有悬垂吲哚环的配合物与没有侧链吲哚环的配合物显着不同,并且吸收光谱和CD光谱表现出对溶剂的依赖性,这与溶液中苯氧基自由基-吲哚的堆积相互作用相符。其他物理化学结果和理论计算结果强烈支持吲哚环作为电子供体通过π-π堆积相互作用稳定苯氧基。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号