当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
9,10-Dihydro-as-indacenodithiophenes: Isomers with an as-Indacene Core
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-03-01 00:00:00 , DOI: 10.1021/acs.joc.8b03049 Hirokazu Miyoshi,Akino Nabe,Shreyam Chatterjee,Yoshito Tobe
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-03-01 00:00:00 , DOI: 10.1021/acs.joc.8b03049 Hirokazu Miyoshi,Akino Nabe,Shreyam Chatterjee,Yoshito Tobe
|
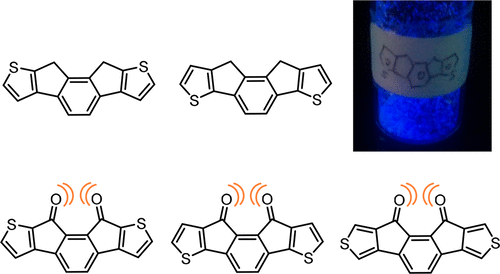
Two isomers of 9,10-dihydro-as-indacenodithiophenes (DIDTs) and the corresponding diketones having an as-indacene core were synthesized. Their thermal, photophysical, and electrochemical properties were investigated, revealing that they depend on the direction of the fusion of the thiophene rings. For the DIDTs, the effect of the mode of ring fusion on the physical properties is discussed by comparison with the previously reported derivatives of DIDT isomers with an s-indacene core. The observed difference between the highest occupied molecular orbital (HOMO)/lowest unoccupied molecular orbital (LUMO) levels of the DIDT isomers is ascribed to the efficiency of π-conjugation, which depends on α- or β-linkage between the terminal thiophenes with the central benzene ring. In addition, the effect of the peripheral aromatic ring (thiophene or benzene) is elucidated by comparison with indeno[2,1-a]fluorene (DIF) bearing an as-indacene core. The HOMO levels of DIDTs are significantly raised compared to that of structurally related DIF because of electron-donating character of the thiophene rings. For the DIDT diketones, structural effect due to the proximate carbonyl groups is discussed by comparison with the isomers with remote carbonyl groups. In diketones bearing proximate carbonyl groups, the LUMO levels are destabilized owing to antibonding interaction between the carbonyl oxygen atoms, resulting in approach of the LUMO and LUMO+1 energy levels.
中文翻译:

9,10- Dihydro- as -indacenodithiophenes:具有as- Indacene核心的异构体
9,10-二氢-的两种异构体作为-indacenodithiophenes(DIDTs)和具有相应的二酮作为-indacene芯合成。研究了它们的热,光物理和电化学性质,发现它们取决于噻吩环的融合方向。对于DIDT,通过与先前报道的带有s的DIDT异构体的衍生物进行比较,讨论了环稠合方式对物理性质的影响。-茚并二烯核。观察到的DIDT异构体的最高占据分子轨道(HOMO)/最低未占据分子轨道(LUMO)水平之间的差异归因于π共轭效率,这取决于末端噻吩与中心苯环。此外,外围芳香环(噻吩或苯)的作用是通过与茚并比较阐明[2,1-一个]芴(DIF)承载有作为-茚并二烯核。由于噻吩环的供电子特性,与结构相关的DIF相比,DIDTs的HOMO水平显着提高。对于DIDT二酮,通过与具有较远羰基的异构体进行比较,讨论了由于邻近羰基引起的结构效应。在带有最接近羰基的二酮中,由于羰基氧原子之间的反键相互作用,LUMO的水平不稳定,从而导致达到LUMO和LUMO + 1的能级。
更新日期:2019-03-01
中文翻译:

9,10- Dihydro- as -indacenodithiophenes:具有as- Indacene核心的异构体
9,10-二氢-的两种异构体作为-indacenodithiophenes(DIDTs)和具有相应的二酮作为-indacene芯合成。研究了它们的热,光物理和电化学性质,发现它们取决于噻吩环的融合方向。对于DIDT,通过与先前报道的带有s的DIDT异构体的衍生物进行比较,讨论了环稠合方式对物理性质的影响。-茚并二烯核。观察到的DIDT异构体的最高占据分子轨道(HOMO)/最低未占据分子轨道(LUMO)水平之间的差异归因于π共轭效率,这取决于末端噻吩与中心苯环。此外,外围芳香环(噻吩或苯)的作用是通过与茚并比较阐明[2,1-一个]芴(DIF)承载有作为-茚并二烯核。由于噻吩环的供电子特性,与结构相关的DIF相比,DIDTs的HOMO水平显着提高。对于DIDT二酮,通过与具有较远羰基的异构体进行比较,讨论了由于邻近羰基引起的结构效应。在带有最接近羰基的二酮中,由于羰基氧原子之间的反键相互作用,LUMO的水平不稳定,从而导致达到LUMO和LUMO + 1的能级。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号