当前位置:
X-MOL 学术
›
Phytochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
4-Hydroxybenzoic acid serves as an endogenous ring precursor for antroquinonol biosynthesis in Antrodia cinnamomea
Phytochemistry ( IF 3.2 ) Pub Date : 2019-05-01 , DOI: 10.1016/j.phytochem.2019.02.011 Kevin Chi-Chung Chou , Hsiang-Lin Wu , Pei-Yin Lin , Shang-Han Yang , Tsu-Liang Chang , Fuu Sheu , Kai-Hsien Chen , Been-Huang Chiang
Phytochemistry ( IF 3.2 ) Pub Date : 2019-05-01 , DOI: 10.1016/j.phytochem.2019.02.011 Kevin Chi-Chung Chou , Hsiang-Lin Wu , Pei-Yin Lin , Shang-Han Yang , Tsu-Liang Chang , Fuu Sheu , Kai-Hsien Chen , Been-Huang Chiang
|
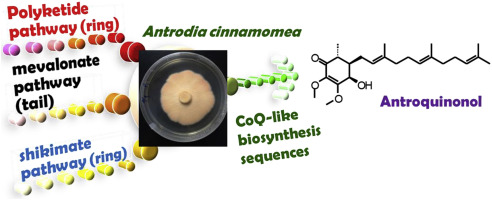
Antrodia cinnamomea, an endemic fungus species of Taiwan, has long been used as a luxurious dietary supplement to enhance liver functions and as a remedy for various cancers. Antroquinonol (AQ), identified from the mycelium of A. cinnamomea, is currently in phase II clinical trials in the USA and Taiwan for the treatment of non-small-cell lung cancer. In the previous studies, we have demonstrated that AQ and 4-acetylantroquinonol B (4-AAQB) utilize orsellinic acid, via polyketide pathway, as the ring precursor, and their biosynthetic sequences are similar to those of coenzyme Q. In order to test 4-hydroxybenzoic acid (4-HBA), synthesized via shikimate pathway, is the ring precursor of AQ analogs, the strategy of metabolic labeling with stable isotopes was applied in this study. Here we have confirmed that 4-HBA serves as the ring precursor for AQ but not a precursor of 4-AAQB. Experimental results indicated that A. cinnamomea preferentially utilizes endogenous 4-HBA via shikimate pathway for AQ biosynthesis. Exogenous tyrosine and phenylalanine can be utilized for AQ biosynthesis when shikimate pathway is blocked by glyphosate. The benzoquinone ring of 4-AAQB is synthesized only via polyketide pathway, but that of AQ is synthesized via both polyketide pathway and shikimate pathway. The precursor-products relationships diagram of AQ and 4-AAQB in A. cinnamomea are proposed based on the experimental findings.
更新日期:2019-05-01





















































 京公网安备 11010802027423号
京公网安备 11010802027423号