当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of the Pyridine Substituent on the Role of the Phenol Functional Group in [Cu(pOHBz)2(dPy)2] Complexes (pOHBz: p‐Hydroxybenzoate, dPy= 4‐Phenylpyridine, 4‐Benzylpyridine, 3‐Phenylpyridine).
ChemistrySelect ( IF 1.9 ) Pub Date : 2017-12-13 , DOI: 10.1002/slct.201702833 Marta Sanchez-Sala 1 , Josefina Pons 1 , Ángel Álvarez-Larena 2 , Concepción Domingo 3 , José A. Ayllón 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2017-12-13 , DOI: 10.1002/slct.201702833 Marta Sanchez-Sala 1 , Josefina Pons 1 , Ángel Álvarez-Larena 2 , Concepción Domingo 3 , José A. Ayllón 1
Affiliation
|
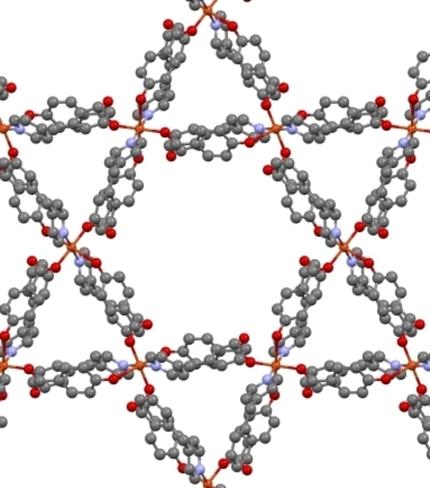
The crystal structure of three new complexes of stoichiometry [Cu(pOHBz)2(dPy)2] (pOHBz: p‐hydroxybenzoate, dPy= 4‐phenylpyridine, 4‐benzylpyridine, 3‐phenylpyridine) and its comparison with the previously reported [Cu(pOHBz)2(Py)2] (1) indicate that the nature and position of the pyridine substituent determines different roles for the phenol group from the pOHBz ligand and, consequently, the coordination number and geometry of Cu(II) centers. Results herein reported show the versatility of the pOHBz ligand towards the coordination of Cu(II). Different carboxylate bonding modes are also observed: monodentate, bidentate chelating or bidentate bridge. Besides, this governs the different roles of the phenol group of the pOHBz ligand: founding of supramolecular hydrogen‐bond based network or direct bonding to the copper cation, yielding 2D coordination polymers.
中文翻译:

吡啶取代基对[Cu(pOHBz)2(dPy)2]配合物中苯酚官能团作用的影响(pOHBz:对羟基苯甲酸酯,dPy = 4-苯基吡啶,4-苄基吡啶,3-苯基吡啶)。
化学计量的三个新配合物[Cu(pOHBz)2(dPy)2 ]的晶体结构(pOHBz:对羟基苯甲酸酯,dPy = 4-苯基吡啶,4-苄基吡啶,3-苯基吡啶)及其与先前报道的[Cu (pOHBz)2(Py)2](1)表示吡啶取代基的性质和位置决定了pOHBz配体对苯酚基团的不同作用,因此决定了Cu(II)中心的配位数和几何形状。本文报道的结果显示了pOHBz配体对Cu(II)配位的多功能性。还观察到了不同的羧酸盐键合模式:单齿,双齿螯合或双齿桥。此外,这支配着pOHBz配体的酚基的不同作用:建立基于超分子氢键的网络或直接键合到铜阳离子上,从而产生2D配位聚合物。
更新日期:2017-12-13
中文翻译:

吡啶取代基对[Cu(pOHBz)2(dPy)2]配合物中苯酚官能团作用的影响(pOHBz:对羟基苯甲酸酯,dPy = 4-苯基吡啶,4-苄基吡啶,3-苯基吡啶)。
化学计量的三个新配合物[Cu(pOHBz)2(dPy)2 ]的晶体结构(pOHBz:对羟基苯甲酸酯,dPy = 4-苯基吡啶,4-苄基吡啶,3-苯基吡啶)及其与先前报道的[Cu (pOHBz)2(Py)2](1)表示吡啶取代基的性质和位置决定了pOHBz配体对苯酚基团的不同作用,因此决定了Cu(II)中心的配位数和几何形状。本文报道的结果显示了pOHBz配体对Cu(II)配位的多功能性。还观察到了不同的羧酸盐键合模式:单齿,双齿螯合或双齿桥。此外,这支配着pOHBz配体的酚基的不同作用:建立基于超分子氢键的网络或直接键合到铜阳离子上,从而产生2D配位聚合物。





























 京公网安备 11010802027423号
京公网安备 11010802027423号