当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct β-Alkylation of Aldehydes via Photoredox Organocatalysis
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2014-04-29 , DOI: 10.1021/ja502639e Jack A Terrett 1 , Michael D Clift , David W C MacMillan
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2014-04-29 , DOI: 10.1021/ja502639e Jack A Terrett 1 , Michael D Clift , David W C MacMillan
Affiliation
|
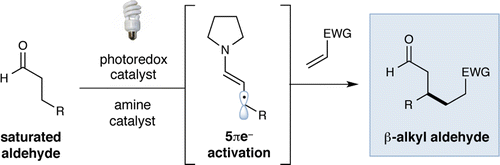
Direct β-alkylation of saturated aldehydes has been accomplished by synergistically combining photoredox catalysis and organocatalysis. Photon-induced enamine oxidation provides an activated β-enaminyl radical intermediate, which readily combines with a wide range of Michael acceptors to produce β-alkyl aldehydes in a highly efficient manner. Furthermore, this redox-neutral, atom-economical C–H functionalization protocol can be achieved both inter- and intramolecularly. Mechanistic studies by various spectroscopic methods suggest that a reductive quenching pathway is operable.
中文翻译:

通过光氧化还原有机催化对醛进行直接 β-烷基化
通过光氧化还原催化和有机催化的协同结合,实现了饱和醛的直接β-烷基化。光子诱导的烯胺氧化提供了一种活化的β-烯胺基自由基中间体,它很容易与各种迈克尔受体结合,以高效的方式产生β-烷基醛。此外,这种氧化还原中性、原子经济的C-H官能化方案可以在分子间和分子内实现。通过各种光谱方法进行的机理研究表明,还原猝灭途径是可行的。
更新日期:2014-04-29
中文翻译:

通过光氧化还原有机催化对醛进行直接 β-烷基化
通过光氧化还原催化和有机催化的协同结合,实现了饱和醛的直接β-烷基化。光子诱导的烯胺氧化提供了一种活化的β-烯胺基自由基中间体,它很容易与各种迈克尔受体结合,以高效的方式产生β-烷基醛。此外,这种氧化还原中性、原子经济的C-H官能化方案可以在分子间和分子内实现。通过各种光谱方法进行的机理研究表明,还原猝灭途径是可行的。





























 京公网安备 11010802027423号
京公网安备 11010802027423号