当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cation–Pi Interaction: A Key Force for Sorption of Fluoroquinolone Antibiotics on Pyrogenic Carbonaceous Materials
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.est.7b02317 Qing Zhao 1, 2 , Siyu Zhang 1, 2 , Xuejiao Zhang 1, 2 , Lei Lei 1, 3 , Wei Ma 1, 3 , Chuanxin Ma 4 , Lei Song 5 , Jingwen Chen 6 , Bo Pan 7 , Baoshan Xing 8
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.est.7b02317 Qing Zhao 1, 2 , Siyu Zhang 1, 2 , Xuejiao Zhang 1, 2 , Lei Lei 1, 3 , Wei Ma 1, 3 , Chuanxin Ma 4 , Lei Song 5 , Jingwen Chen 6 , Bo Pan 7 , Baoshan Xing 8
Affiliation
|
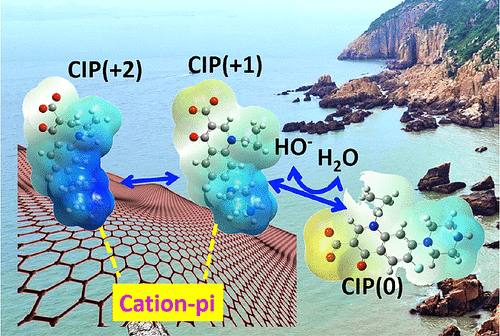
Cation–pi attraction is a major force that determines macromolecular structures and drug-receptor interactions. However, the role of the cation–pi interaction in sorption of fluoroquinolone antibiotics by pyrogenic carbonaceous materials (PCMs) has not been addressed. We studied sorption of ciprofloxacin (CIP) on graphite to quantify the contribution of the cation–pi interaction. Through competition experiments, the decreased amount of sorbed CIP by sequential treatment with hexadecane, phenanthrene and benzylamine represents the contribution of hydrophobic, pi-pi and cation–pi interactions, respectively. Benzylamine competed more strongly with CIP than n-hexadecane and phenanthrene, indicating that cation–pi is a major force. Cation–pi interactions accounted for up to 72.6% of the total sorption at an initial CIP concentration of 0.000015 mmol/L. Importantly, species transformation (CIP(0) captures H+ from water to form CIP(+1)) induced by cation-pi interactions was verified both experimentally and theoretically and can be used to explain the environmental behavior of other fluoroquinolone antibiotics and biochemical processes of amino acids that interact with aromatic moieties. Because of the significant role of cation–pi interactions, CIP desorption increased up to 2.32 times when Na+ increased from 0.01 mM to 0.45 mM, which is an environmentally relevant scenario at river estuaries. Hence, behaviors of fluoroquinolone antibiotics that are affected by ionic strength changes need to be carefully evaluated, especially in river estuaries.
中文翻译:

阳离子-Pi相互作用:氟喹诺酮类抗生素在热解碳质材料上的吸附关键力量
阳离子-π吸引是决定大分子结构和药物-受体相互作用的主要力量。但是,阳离子-π相互作用在热解碳质材料(PCM)吸附氟喹诺酮抗生素中的作用尚未得到解决。我们研究了环丙沙星(CIP)在石墨上的吸附,以量化阳离子-π相互作用的作用。通过竞争实验,通过依次用十六烷,菲和苄胺处理减少的CIP吸附量分别代表疏水作用,pi-pi和阳离子-pi相互作用。苄胺在CIP上的竞争比n-十六烷和菲,表明阳离子-pi是主要力量。在初始CIP浓度为0.000015 mmol / L时,阳离子与pi的相互作用占总吸附量的72.6%。重要的是,通过阳离子-π相互作用诱导的物种转化(CIP(0)从水中捕获H +形成CIP(+1))已通过实验和理论验证,可用于解释其他氟喹诺酮类抗生素的环境行为和生化过程与芳香族部分相互作用的氨基酸。由于阳离子与pi相互作用的重要作用,当Na +从0.01 mM增加到0.45 mM,这在河口地区与环境有关。因此,需要仔细评估受离子强度变化影响的氟喹诺酮类抗生素的行为,尤其是在河口。
更新日期:2017-11-19
中文翻译:

阳离子-Pi相互作用:氟喹诺酮类抗生素在热解碳质材料上的吸附关键力量
阳离子-π吸引是决定大分子结构和药物-受体相互作用的主要力量。但是,阳离子-π相互作用在热解碳质材料(PCM)吸附氟喹诺酮抗生素中的作用尚未得到解决。我们研究了环丙沙星(CIP)在石墨上的吸附,以量化阳离子-π相互作用的作用。通过竞争实验,通过依次用十六烷,菲和苄胺处理减少的CIP吸附量分别代表疏水作用,pi-pi和阳离子-pi相互作用。苄胺在CIP上的竞争比n-十六烷和菲,表明阳离子-pi是主要力量。在初始CIP浓度为0.000015 mmol / L时,阳离子与pi的相互作用占总吸附量的72.6%。重要的是,通过阳离子-π相互作用诱导的物种转化(CIP(0)从水中捕获H +形成CIP(+1))已通过实验和理论验证,可用于解释其他氟喹诺酮类抗生素的环境行为和生化过程与芳香族部分相互作用的氨基酸。由于阳离子与pi相互作用的重要作用,当Na +从0.01 mM增加到0.45 mM,这在河口地区与环境有关。因此,需要仔细评估受离子强度变化影响的氟喹诺酮类抗生素的行为,尤其是在河口。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号