当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
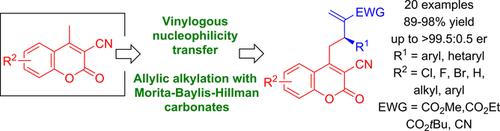
Vinylogous Nucleophiles Bearing the Endocyclic Double Bond in the Allylic Alkylation with Morita‐Baylis‐Hillman Carbonates
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-10-25 , DOI: 10.1002/adsc.201701185 Dorota Kowalczyk 1 , Łukasz Albrecht 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-10-25 , DOI: 10.1002/adsc.201701185 Dorota Kowalczyk 1 , Łukasz Albrecht 1
Affiliation
|
This study demonstrates that vinylogous transfer of nucleophilicity in the allylic alkylation with Morita‐Baylis‐Hillman carbonates can be accomplished through the endocyclic double bond in 3‐cyano‐4‐methylcoumarins. The developed reaction provides a straightforward access to functionalized coumarin derivatives of biological and synthetic relevance. Target, highly functionalized products have been chemoselectively and efficiently obtained in very high yield (up to 98%) and with excellent enantioselectivity (up to 99.5:0.5 er).
中文翻译:

具有Morita-Baylis-Hillman碳酸盐的烯丙基烷基化中带有环内双键的长核亲核试剂
这项研究表明,通过Morita-Baylis-Hillman碳酸酯进行的烯丙基烷基化中的亲核性的乙烯基转移可以通过3-氰基-4-甲基香豆素中的内环双键来完成。发达的反应提供了直接获得具有生物学和合成相关性的功能化香豆素衍生物的途径。以非常高的收率(高达98%)和优异的对映选择性(高达99.5:0.5 er),以化学选择性和有效的方式获得了目标高度功能化的产品。
更新日期:2017-10-25
中文翻译:

具有Morita-Baylis-Hillman碳酸盐的烯丙基烷基化中带有环内双键的长核亲核试剂
这项研究表明,通过Morita-Baylis-Hillman碳酸酯进行的烯丙基烷基化中的亲核性的乙烯基转移可以通过3-氰基-4-甲基香豆素中的内环双键来完成。发达的反应提供了直接获得具有生物学和合成相关性的功能化香豆素衍生物的途径。以非常高的收率(高达98%)和优异的对映选择性(高达99.5:0.5 er),以化学选择性和有效的方式获得了目标高度功能化的产品。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号